1. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016; 387:1377–1396. PMID:
27115820.
2. Ansari S, Haboubi H, Haboubi N. Adult obesity complications: challenges and clinical impact. Ther Adv Endocrinol Metab. 2020; 11:2042018820934955. PMID:
32612803.
3. Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015; 33:673–689. PMID:
25471927.
4. Larsen SC, Ängquist L, Sørensen TI, Heitmann BL. 24h urinary sodium excretion and subsequent change in weight, waist circumference and body composition. PLoS One. 2013; 8:e69689. PMID:
23936079.
5. Yi SS, Firestone MJ, Beasley JM. Independent associations of sodium intake with measures of body size and predictive body fatness. Obesity (Silver Spring). 2015; 23:20–23. PMID:
25294369.
6. Yoon YS, Oh SW. Sodium density and obesity; the Korea National Health and Nutrition Examination Survey 2007-2010. Eur J Clin Nutr. 2013; 67:141–146. PMID:
23249877.
7. McLean RM. Measuring population sodium intake: a review of methods. Nutrients. 2014; 6:4651–4662. PMID:
25353661.
8. Grimes CA, Bolhuis DP, He FJ, Nowson CA. Dietary sodium intake and overweight and obesity in children and adults: a protocol for a systematic review and meta-analysis. Syst Rev. 2016; 5:7. PMID:
26781844.
9. Whiting P, Savović J, Higgins JP, Caldwell DM, Reeves BC, Shea B, Davies P, Kleijnen J, Churchill R. ROBIS group. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016; 69:225–234. PMID:
26092286.
10. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560. PMID:
12958120.
11. Grimes CA, Bolton KA, Booth AB, Khokhar D, Service C, He FH, Nowson CA. The association between dietary sodium intake, adiposity and sugar-sweetened beverages in children and adults: a systematic review and meta-analysis. Br J Nutr. 2021; 126:409–427. PMID:
33054868.
12. Moosavian SP, Haghighatdoost F, Surkan PJ, Azadbakht L. Salt and obesity: a systematic review and meta-analysis of observational studies. Int J Food Sci Nutr. 2017; 68:265–277. PMID:
27706955.
13. Kang YJ, Wang HW, Cheon SY, Lee HJ, Hwang KM, Yoon HS. Associations of obesity and dyslipidemia with intake of sodium, fat, and sugar among Koreans: a qualitative systematic review. Clin Nutr Res. 2016; 5:290–304. PMID:
27812518.
14. Baudrand R, Campino C, Carvajal CA, Olivieri O, Guidi G, Faccini G, Vöhringer PA, Cerda J, Owen G, Kalergis AM, et al. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin Endocrinol (Oxf). 2014; 80:677–684. PMID:
23594269.
15. Hoffmann IS, Cubeddu LX. Salt and the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2009; 19:123–128. PMID:
18556187.
16. Hulthén L, Aurell M, Klingberg S, Hallenberg E, Lorentzon M, Ohlsson C. Salt intake in young Swedish men. Public Health Nutr. 2010; 13:601–605. PMID:
19968896.
17. Ma Y, He FJ, MacGregor GA. High salt intake: independent risk factor for obesity? Hypertension. 2015; 66:843–849. PMID:
26238447.
18. Madhavan S, Alderman MH. Ethnicity and the relationship of sodium intake to blood pressure. J Hypertens. 1994; 12:97–103. PMID:
8157951.
19. Nam GE, Kim SM, Choi MK, Heo YR, Hyun TS, Lyu ES, Oh SY, Park HR, Ro HK, Han K, et al. Association between 24-h urinary sodium excretion and obesity in Korean adults: a multicenter study. Nutrition. 2017; 41:113–119. PMID:
28760420.
20. Polonia JJ, Magalhaes MT, Senra D, Barbosa L, Silva JA, Ribeiro SM. Association of 24-h urinary salt excretion with central haemodynamics and assessment of food categories contributing to salt consumption in Portuguese patients with hypertension. Blood Press Monit. 2013; 18:303–310. PMID:
24192843.
21. Vega-Vega O, Fonseca-Correa JI, Mendoza-De la Garza A, Rincón-Pedrero R, Espinosa-Cuevas A, Baeza-Arias Y, Dary O, Herrero-Bervera B, Nieves-Anaya I, Correa-Rotter R. Contemporary dietary intake: too much sodium, not enough potassium, yet sufficient iodine: the SALMEX Cohort results. Nutrients. 2018; 10:816. PMID:
29941792.
22. Verhave JC, Hillege HL, Burgerhof JG, Janssen WM, Gansevoort RT, Navis GJ, de Zeeuw D, de Jong PE. PREVEND Study Group. Sodium intake affects urinary albumin excretion especially in overweight subjects. J Intern Med. 2004; 256:324–330. PMID:
15367175.
23. Yan L, Guo X, Wang H, Zhang J, Tang J, Lu Z, Cai X, Liu L, Gracely EJ, Ma J. Population-based association between urinary excretion of sodium, potassium and its ratio with albuminuria in Chinese. Asia Pac J Clin Nutr. 2016; 25:785–797. PMID:
27702722.
24. Rhee MY, Kim JH, Kim YS, Chung JW, Bae JH, Nah DY, Kim YK, Lee MM, Lim CY, Byun JE, et al. High sodium intake in women with metabolic syndrome. Korean Circ J. 2014; 44:30–36. PMID:
24497887.
25. Han SY, Hong JW, Noh JH, Kim DJ. Association of the estimated 24-h urinary sodium excretion with albuminuria in adult Koreans: the 2011 Korea National Health and Nutrition Examination Survey. PLoS One. 2014; 9:e109073. PMID:
25313865.
26. Han W, Hu Y, Tang Y, Xue F, Hou L, Liang S, Zhang B, Wang W, Asaiti K, Pang H, et al. Relationship between urinary sodium with blood pressure and hypertension among a Kazakh community population in Xinjiang, China. J Hum Hypertens. 2017; 31:333–340. PMID:
28054572.
27. Huh JH, Lim JS, Lee MY, Chung CH, Shin JY. Gender-specific association between urinary sodium excretion and body composition: analysis of the 2008-2010 Korean National Health and Nutrition Examination Surveys. Metabolism. 2015; 64:837–844. PMID:
25873364.
28. Lee SK, Kim JS, Kim SH, Kim YH, Lim HE, Kim EJ, Park CG, Cho GY, Kim J, Baik I, et al. Sodium excretion and cardiovascular structure and function in the nonhypertensive population: the Korean Genome and Epidemiology Study. Am J Hypertens. 2015; 28:1010–1016. PMID:
25534867.
29. Oh SW, Han KH, Han SY, Koo HS, Kim S, Chin HJ. Association of sodium excretion with metabolic syndrome, insulin resistance, and body fat. Medicine (Baltimore). 2015; 94:e1650. PMID:
26426658.
30. Petermann-Rocha F, Sillars A, Brown R, Sweeney L, Troncoso C, García-Hermoso A, Leiva AM, Martínez MA, Diaz-Martínez X, Poblete-Valderrama F, et al. Sociodemographic patterns of urine sodium excretion and its association with hypertension in Chile: a cross-sectional analysis. Public Health Nutr. 2019; 22:2012–2021. PMID:
30761970.
31. Watanabe S, Konta T, Ichikawa K, Watanabe M, Ishizawa K, Ueno Y, Yamashita H, Kayama T, Kubota I. The association between urinary sodium excretion and blood pressure in a community-based population: the Yamagata (Takahata) study. Clin Exp Nephrol. 2019; 23:380–386. PMID:
30293215.
32. Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw KT. Estimated urinary sodium excretion and risk of heart failure in men and women in the EPIC-Norfolk study. Eur J Heart Fail. 2014; 16:394–402. PMID:
24464931.
33. Welsh CE, Welsh P, Jhund P, Delles C, Celis-Morales C, Lewsey JD, Gray S, Lyall D, Iliodromiti S, Gill JM, et al. Urinary sodium excretion, blood pressure, and risk of future cardiovascular disease and mortality in subjects without prior cardiovascular disease. Hypertension. 2019; 73:1202–1209. PMID:
31067194.
34. Eufinger SC, Votaw J, Faber T, Ziegler TR, Goldberg J, Bremner JD, Vaccarino V. Habitual dietary sodium intake is inversely associated with coronary flow reserve in middle-aged male twins. Am J Clin Nutr. 2012; 95:572–579. PMID:
22258268.
35. Jablonski KL, Gates PE, Pierce GL, Seals DR. Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure. Ther Adv Cardiovasc Dis. 2009; 3:347–356. PMID:
19723834.
36. Murata A, Fujino Y, Pham TM, Kubo T, Mizoue T, Tokui N, Matsuda S, Yoshimura T. Prospective cohort study evaluating the relationship between salted food intake and gastrointestinal tract cancer mortality in Japan. Asia Pac J Clin Nutr. 2010; 19:564–571. PMID:
21147719.
37. Radhika G, Sathya RM, Sudha V, Ganesan A, Mohan V. Dietary salt intake and hypertension in an urban south Indian population--[CURES - 53]. J Assoc Physicians India. 2007; 55:405–411. PMID:
17879493.
38. Sharma S, McFann K, Chonchol M, Kendrick J. Dietary sodium and potassium intake is not associated with elevated blood pressure in US adults with no prior history of hypertension. J Clin Hypertens (Greenwich). 2014; 16:418–423. PMID:
24720647.
39. Shikata K, Kiyohara Y, Kubo M, Yonemoto K, Ninomiya T, Shirota T, Tanizaki Y, Doi Y, Tanaka K, Oishi Y, et al. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. Int J Cancer. 2006; 119:196–201. PMID:
16450397.
40. Zhao X, Yin X, Li X, Yan LL, Lam CT, Li S, He F, Xie W, Sang B, Luobu G, et al. Using a low-sodium, high-potassium salt substitute to reduce blood pressure among Tibetans with high blood pressure: a patient-blinded randomized controlled trial. PLoS One. 2014; 9:e110131. PMID:
25338053.
41. Yokokawa H, Yuasa M, Nedsuwan S, Moolphate S, Fukuda H, Kitajima T, Minematsu K, Tanimura S, Marui E. Daily salt intake estimated by overnight urine collections indicates a high cardiovascular disease risk in Thailand. Asia Pac J Clin Nutr. 2016; 25:39–45. PMID:
26965760.
42. Zhao L, Cogswell ME, Yang Q, Zhang Z, Onufrak S, Jackson SL, Chen TC, Loria CM, Wang CY, Wright JD, et al. Association of usual 24-h sodium excretion with measures of adiposity among adults in the United States: NHANES, 2014. Am J Clin Nutr. 2019; 109:139–147. PMID:
30624582.
43. Song HJ, Cho YG, Lee HJ. Dietary sodium intake and prevalence of overweight in adults. Metabolism. 2013; 62:703–708. PMID:
23357528.
44. Ge Z, Guo X, Chen X, Tang J, Yan L, Ren J, Zhang J, Lu Z, Dong J, Xu J, et al. Association between 24 h urinary sodium and potassium excretion and the metabolic syndrome in Chinese adults: the Shandong and Ministry of Health Action on Salt and Hypertension (SMASH) study. Br J Nutr. 2015; 113:996–1002. PMID:
25743698.
45. Zhang X, Wang J, Li J, Yu Y, Song Y. A positive association between dietary sodium intake and obesity and central obesity: results from the National Health and Nutrition Examination Survey 1999-2006. Nutr Res. 2018; 55:33–44. PMID:
29914626.
46. Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med. 2001; 161:685–693. PMID:
11231700.
47. Bulpitt CJ, Daymond M, Bulpitt PF, Ferrier G, Harrison R, Lewis PJ, Dollery CT. Is low salt dietary advice a useful therapy in hypertensive patients with poorly controlled blood pressure? Ann Clin Res. 1984; 16(Suppl 43):143–149. PMID:
6398984.
48. Geleijnse JM, Witteman JC, Bak AA, den Breeijen JH, Grobbee DE. Reduction in blood pressure with a low sodium, high potassium, high magnesium salt in older subjects with mild to moderate hypertension. BMJ. 1994; 309:436–440. PMID:
7920126.
49. Kumanyika SK, Hebert PR, Cutler JA, Lasser VI, Sugars CP, Steffen-Batey L, Brewer AA, Cameron M, Shepek LD, Cook NR, et al. Feasibility and efficacy of sodium reduction in the Trials of Hypertension Prevention, phase I. Hypertension. 1993; 22:502–512. PMID:
8406655.
50. Nowson CA, Wattanapenpaiboon N, Pachett A. Low-sodium dietary approaches to stop hypertension-type diet including lean red meat lowers blood pressure in postmenopausal women. Nutr Res. 2009; 29:8–18. PMID:
19185772.
51. Petersen KS, Torpy DJ, Chapman IM, Guha S, Clifton PM, Turner K, Keogh JB. Food label education does not reduce sodium intake in people with type 2 diabetes mellitus. A randomised controlled trial. Appetite. 2013; 68:147–151. PMID:
23665299.
52. Beard TC, Cooke HM, Gray WR, Barge R. Randomised controlled trial of a no-added-sodium diet for mild hypertension. Lancet. 1982; 2:455–458. PMID:
6125636.
53. Dodson PM, Beevers M, Hallworth R, Webberley MJ, Fletcher RF, Taylor KG. Sodium restriction and blood pressure in hypertensive type II diabetics: randomised blind controlled and crossover studies of moderate sodium restriction and sodium supplementation. BMJ. 1989; 298:227–230. PMID:
2493869.
54. Gilleran G, O’Leary M, Bartlett WA, Vinall H, Jones AF, Dodson PM. Effects of dietary sodium substitution with potassium and magnesium in hypertensive type II diabetics: a randomised blind controlled parallel study. J Hum Hypertens. 1996; 10:517–521. PMID:
8895035.
55. He FJ, Wu Y, Feng XX, Ma J, Ma Y, Wang H, Zhang J, Yuan J, Lin CP, Nowson C, et al. School based education programme to reduce salt intake in children and their families (School-EduSalt): cluster randomised controlled trial. BMJ. 2015; 350:h770. PMID:
25788018.
56. Nouvenne A, Meschi T, Prati B, Guerra A, Allegri F, Vezzoli G, Soldati L, Gambaro G, Maggiore U, Borghi L. Effects of a low-salt diet on idiopathic hypercalciuria in calcium-oxalate stone formers: a 3-mo randomized controlled trial. Am J Clin Nutr. 2010; 91:565–570. PMID:
20042524.
57. Hypertension Prevention Trial Research Group. The Hypertension Prevention Trial: three-year effects of dietary changes on blood pressure. Arch Intern Med. 1990; 150:153–162. PMID:
2404477.
58. Staessen J, Bulpitt CJ, Fagard R, Joossens JV, Lijnen P, Amery A. Salt intake and blood pressure in the general population: a controlled intervention trial in two towns. J Hypertens. 1988; 6:965–973. PMID:
3065411.
59. Takahashi Y, Sasaki S, Okubo S, Hayashi M, Tsugane S. Blood pressure change in a free-living population-based dietary modification study in Japan. J Hypertens. 2006; 24:451–458. PMID:
16467647.
60. The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. Arch Intern Med. 1997; 157:657–667. PMID:
9080920.
61. Ard JD, Coffman CJ, Lin PH, Svetkey LP. One-year follow-up study of blood pressure and dietary patterns in dietary approaches to stop hypertension (DASH)-sodium participants. Am J Hypertens. 2004; 17:1156–1162. PMID:
15607623.
62. Sakaki M, Tsuchihashi T, Arakawa K. Characteristics of the hypertensive patients with good and poor compliance to long-term salt restriction. Clin Exp Hypertens. 2014; 36:92–96. PMID:
24625335.
63. Takahashi Y, Sasaki S, Okubo S, Hayashi M, Tsugane S. Maintenance of a low-sodium, high-carotene and -vitamin C diet after a 1-year dietary intervention: the Hiraka dietary intervention follow-up study. Prev Med. 2006; 43:14–19. PMID:
16707149.
64. Kim J, Lim G, Kang S, Lee K, Park T, Kim J. The relationship between daily sodium intake and obesity in Korean adults. Korean J Health Promot. 2015; 15:175–184.
65. Lim S, Yang S. Association between dietary sodium intake and abdominal obesity in pre-diabetes Korean adults. J Korean Soc Food Sci Nutr. 2014; 43:763–771.
66. Oh H, Kim H, Jun D, Lee S. Associations between 24-hour urine sodium excretion level and obesity-related metabolic risk factors. Korean J Community Nutr. 2015; 20:460–467.
67. Lee M, Sorn SR, Lee Y, Kang I. Salt induces adipogenesis/lipogenesis and inflammatory adipocytokines secretion in adipocytes. Int J Mol Sci. 2019; 20:160. PMID:
30621146.
68. Lanaspa MA, Kuwabara M, Andres-Hernando A, Li N, Cicerchi C, Jensen T, Orlicky DJ, Roncal-Jimenez CA, Ishimoto T, Nakagawa T, et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc Natl Acad Sci U S A. 2018; 115:3138–3143. PMID:
29507217.
69. Fonseca-Alaniz MH, Takada J, Andreotti S, de Campos TB, Campaña AB, Borges-Silva CN, Lima FB. High sodium intake enhances insulin-stimulated glucose uptake in rat epididymal adipose tissue. Obesity (Silver Spring). 2008; 16:1186–1192. PMID:
18369340.
70. Fonseca-Alaniz MH, Brito LC, Borges-Silva CN, Takada J, Andreotti S, Lima FB. High dietary sodium intake increases white adipose tissue mass and plasma leptin in rats. Obesity (Silver Spring). 2007; 15:2200–2208. PMID:
17890487.
71. Zhang Y, Li F, Liu FQ, Chu C, Wang Y, Wang D, Guo TS, Wang JK, Guan GC, Ren KY, et al. Elevation of fasting ghrelin in healthy human subjects consuming a high-salt diet: a novel mechanism of obesity? Nutrients. 2016; 8:323.
72. Mähler A, Klamer S, Maifeld A, Bartolomaeus H, Markó L, Chen CY, Forslund SK, Boschmann M, Müller DN, Wilck N. Increased salt intake decreases diet-induced thermogenesis in healthy volunteers: a randomized placebo-controlled study. Nutrients. 2022; 14:253. PMID:
35057434.
73. Cogswell ME, Maalouf J, Elliott P, Loria CM, Patel S, Bowman BA. Use of urine biomarkers to assess sodium intake: challenges and opportunities. Annu Rev Nutr. 2015; 35:349–387. PMID:
25974702.
74. Campbell NR, He FJ, Tan M, Cappuccio FP, Neal B, Woodward M, Cogswell ME, McLean R, Arcand J, MacGregor G, et al. The International Consortium for Quality Research on Dietary Sodium/Salt (TRUE) position statement on the use of 24-hour, spot, and short duration (<24 hours) timed urine collections to assess dietary sodium intake. J Clin Hypertens (Greenwich). 2019; 21:700–709. PMID:
31087778.
75. Bie P. Mechanisms of sodium balance: total body sodium, surrogate variables, and renal sodium excretion. Am J Physiol Regul Integr Comp Physiol. 2018; 315:R945–R962. PMID:
30110176.
76. Huang L, Crino M, Wu JH, Woodward M, Barzi F, Land MA, McLean R, Webster J, Enkhtungalag B, Neal B. Mean population salt intake estimated from 24-h urine samples and spot urine samples: a systematic review and meta-analysis. Int J Epidemiol. 2016; 45:239–250. PMID:
26796216.
77. Ji C, Sykes L, Paul C, Dary O, Legetic B, Campbell NR, Cappuccio FP. Sub-Group for Research and Surveillance of the PAHO–WHO Regional Expert Group for Cardiovascular Disease Prevention Through Population-wide Dietary Salt Reduction. Systematic review of studies comparing 24-hour and spot urine collections for estimating population salt intake. Rev Panam Salud Publica. 2012; 32:307–315. PMID:
23299293.
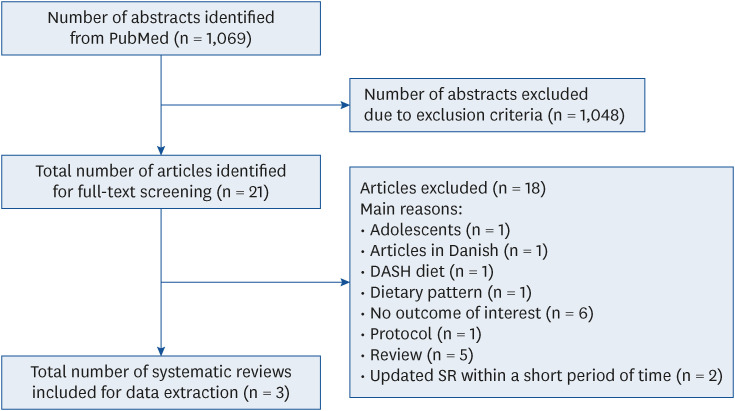
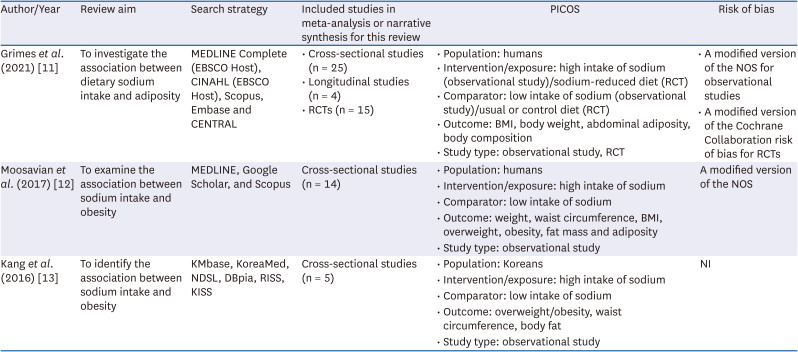
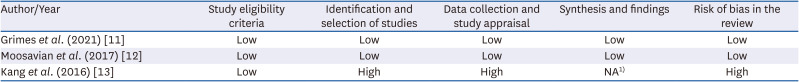
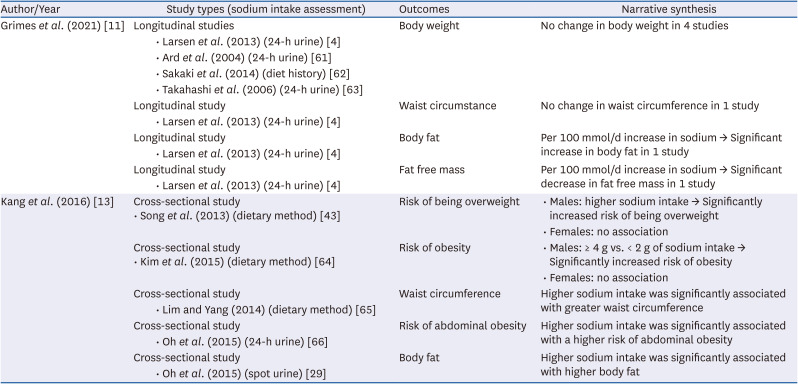




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download