Vancomycin is a glycopeptide antibiotic widely used for the treatment of skin and soft tissue infection, endocarditis, pneumonia, bone and joint infection, and central nervous system infections caused by methicillin-resistant
Staphylococcus aureus (MRSA).
123 One of the major concerns when using vancomycin is the occurrence of acute kidney injury (AKI).
4 The incidence of AKI associated with the use of vancomycin ranges from 12% to 43%, and patients with higher exposure to vancomycin are more likely to experience AKI. The incidence of AKI and the narrow therapeutic index of the drug necessitated therapeutic drug monitoring (TDM).
15 The pharmacodynamic parameter currently thought to be the best predictor of effective vancomycin therapeutic activity is the area under the curve over 24 hours to the minimum inhibitory concentration (AUC
0-24/MIC).
6
The 2020 revised consensus guideline for the use and TDM of vancomycin suggests a target AUC
0-24/MIC of 400 to 600 (assuming an MIC of 1 mg/L) for serious MRSA infections.
78 The guideline also suggests the collection of two concentrations (trough [C
min] and peak [C
max] concentrations) at near steady-state for optimal TDM. The therapeutic effectiveness and safety of the new AUC
0-24-based TDM has not yet been explored in detail, and its effect on the incidence of AKI in the clinical setting has yet to be investigated. In this study, we evaluated the predictability of AKI by vancomycin AUC
0-24, trough levels and TDM estimation methods and analyzed the cutoff values of AUC
0-24 for the prediction of AKI.
This study was a 1-year, retrospective, single-center study to evaluate the pharmacokinetics and incidence of AKI among patients who underwent TDM from January 2020 to December 2020 at the Seoul National University Bundang Hospital. To be included in the study, patients were required to have at least two quantifiable vancomycin concentrations and baseline (measured between −28 and −7 days from the TDM consultation date) estimated glomerular filtration rates (eGFRs) exceeding 60 mL/min/1.73 m
2. TDM data were considered as AKI group if the post-TDM creatinine value was either more than 1.5 times that of the baseline value and/or if more than 0.3 mg/dL increase in absolute value was observed.
910
The AUC0-24 and trough values were calculated by both one- and two-point sampling methods. AUC0-24 cutoff values of 500, 600, and 700 mg*h/L and trough cutoff values of 15 and 20 µg/mL were evaluated for the predictability of AKI. Receiver operating characteristic (ROC) analysis was performed to evaluate the threshold value of AUC0-24 and trough concentration for the best prediction of the incidence of AKI. Multivariable logistic regression analysis was performed to evaluate the calculated threshold values. Apart from the vancomycin exposure parameters, risk factors that were significant at P = 0.2 in univariate analysis were evaluated in the model. Backward elimination was performed until only variables with P ≤ 0.05 remained. Adjusted odds ratio values were calculated to present the relationship between variables and AKI. All statistical analyses were performed using SAS software version 9.4 (SAS institute, Cary, NC, USA) and Statistical Package R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
A total of 407 patient’s TDM data which met the inclusion criteria were identified between January 2020 and December 2020. Among them, 161 TDM cases were included in the analysis (
Supplementary Fig. 1). Following patients were excluded from the analysis: no creatinine data were available on baseline and/or at the TDM report time (n = 156); AUC and/or trough cannot be calculated by standard method (n = 23); or less than three consecutive doses of vancomycin were administered and/or less than two quantifiable concentrations were available (n = 67). The average vancomycin administration time before TDM consultation were: 8.54 ± 3.52 days and 7.18 ± 0.40 days for no AKI group and AKI group, respectively. Among the cases included in the analysis, AKI was observed in 11 (6.8%) patients (
Table 1).
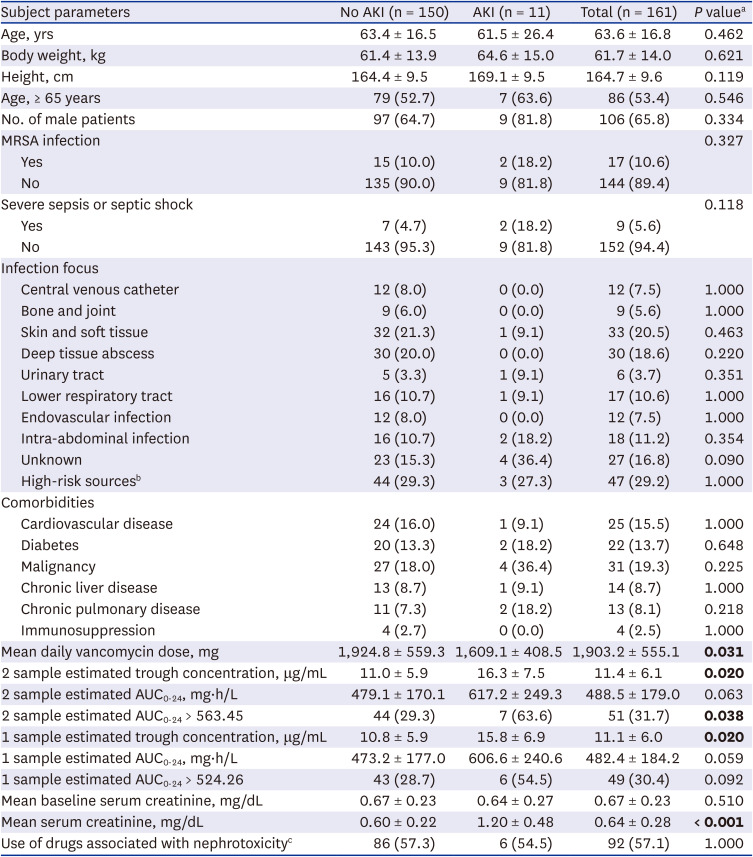
Table 1
Demographic data and base characteristics
|
Subject parameters |
No AKI (n = 150) |
AKI (n = 11) |
Total (n = 161) |
P valuea
|
|
Age, yrs |
63.4 ± 16.5 |
61.5 ± 26.4 |
63.6 ± 16.8 |
0.462 |
|
Body weight, kg |
61.4 ± 13.9 |
64.6 ± 15.0 |
61.7 ± 14.0 |
0.621 |
|
Height, cm |
164.4 ± 9.5 |
169.1 ± 9.5 |
164.7 ± 9.6 |
0.119 |
|
Age, ≥ 65 years |
79 (52.7) |
7 (63.6) |
86 (53.4) |
0.546 |
|
No. of male patients |
97 (64.7) |
9 (81.8) |
106 (65.8) |
0.334 |
|
MRSA infection |
|
|
|
0.327 |
|
Yes |
15 (10.0) |
2 (18.2) |
17 (10.6) |
|
No |
135 (90.0) |
9 (81.8) |
144 (89.4) |
|
Severe sepsis or septic shock |
|
|
|
0.118 |
|
Yes |
7 (4.7) |
2 (18.2) |
9 (5.6) |
|
No |
143 (95.3) |
9 (81.8) |
152 (94.4) |
|
Infection focus |
|
|
|
|
|
Central venous catheter |
12 (8.0) |
0 (0.0) |
12 (7.5) |
1.000 |
|
Bone and joint |
9 (6.0) |
0 (0.0) |
9 (5.6) |
1.000 |
|
Skin and soft tissue |
32 (21.3) |
1 (9.1) |
33 (20.5) |
0.463 |
|
Deep tissue abscess |
30 (20.0) |
0 (0.0) |
30 (18.6) |
0.220 |
|
Urinary tract |
5 (3.3) |
1 (9.1) |
6 (3.7) |
0.351 |
|
Lower respiratory tract |
16 (10.7) |
1 (9.1) |
17 (10.6) |
1.000 |
|
Endovascular infection |
12 (8.0) |
0 (0.0) |
12 (7.5) |
1.000 |
|
Intra-abdominal infection |
16 (10.7) |
2 (18.2) |
18 (11.2) |
0.354 |
|
Unknown |
23 (15.3) |
4 (36.4) |
27 (16.8) |
0.090 |
|
High-risk sourcesb
|
44 (29.3) |
3 (27.3) |
47 (29.2) |
1.000 |
|
Comorbidities |
|
|
|
|
|
Cardiovascular disease |
24 (16.0) |
1 (9.1) |
25 (15.5) |
1.000 |
|
Diabetes |
20 (13.3) |
2 (18.2) |
22 (13.7) |
0.648 |
|
Malignancy |
27 (18.0) |
4 (36.4) |
31 (19.3) |
0.225 |
|
Chronic liver disease |
13 (8.7) |
1 (9.1) |
14 (8.7) |
1.000 |
|
Chronic pulmonary disease |
11 (7.3) |
2 (18.2) |
13 (8.1) |
0.218 |
|
Immunosuppression |
4 (2.7) |
0 (0.0) |
4 (2.5) |
1.000 |
|
Mean daily vancomycin dose, mg |
1,924.8 ± 559.3 |
1,609.1 ± 408.5 |
1,903.2 ± 555.1 |
0.031
|
|
2 sample estimated trough concentration, μg/mL |
11.0 ± 5.9 |
16.3 ± 7.5 |
11.4 ± 6.1 |
0.020
|
|
2 sample estimated AUC0-24, mg∙h/L |
479.1 ± 170.1 |
617.2 ± 249.3 |
488.5 ± 179.0 |
0.063 |
|
2 sample estimated AUC0-24 > 563.45 |
44 (29.3) |
7 (63.6) |
51 (31.7) |
0.038
|
|
1 sample estimated trough concentration, μg/mL |
10.8 ± 5.9 |
15.8 ± 6.9 |
11.1 ± 6.0 |
0.020
|
|
1 sample estimated AUC0-24, mg∙h/L |
473.2 ± 177.0 |
606.6 ± 240.6 |
482.4 ± 184.2 |
0.059 |
|
1 sample estimated AUC0-24 > 524.26 |
43 (28.7) |
6 (54.5) |
49 (30.4) |
0.092 |
|
Mean baseline serum creatinine, mg/dL |
0.67 ± 0.23 |
0.64 ± 0.27 |
0.67 ± 0.23 |
0.510 |
|
Mean serum creatinine, mg/dL |
0.60 ± 0.22 |
1.20 ± 0.48 |
0.64 ± 0.28 |
< 0.001
|
|
Use of drugs associated with nephrotoxicityc
|
86 (57.3) |
6 (54.5) |
92 (57.1) |
1.000 |

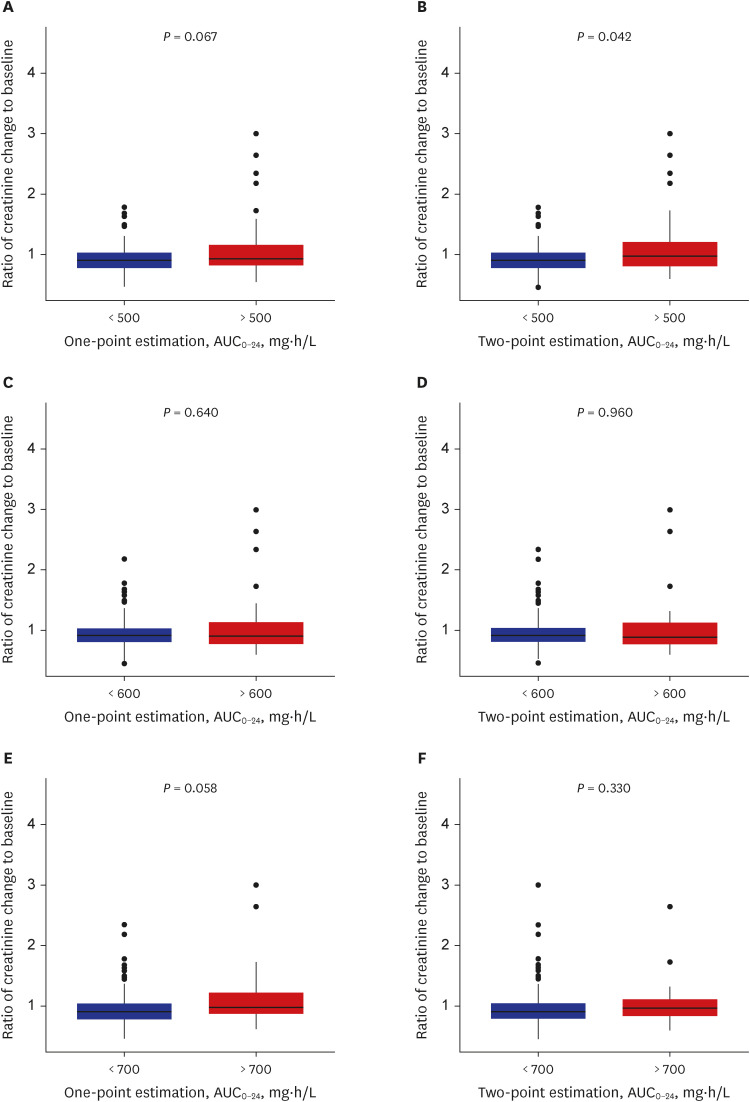
Among the analyzed TDM data, a positive correlation between vancomycin AUC
0-24 and the ratio of creatinine change was observed (
Supplementary Fig. 2). The estimated AUC
0-24 values from both estimation methods were comparable (
Supplementary Fig. 3), and trough levels in both the one- and two-point estimation methods were well correlated with the increase in 24-hour AUC (
Supplementary Fig. 4). When AUC
0-24 cutoff values were evaluated for the correlation between the ratio of creatinine change, only the value of 500 mg*h/L in the two sampling methods was significantly associated with an increase in the creatinine ratio (
P = 0.042, Mann–Whitney
U test) (
Fig. 1). Comparison of trough cutoff values showed comparable predictability of the ratio of creatinine change across all comparisons (
Supplementary Fig. 5).
Fig. 1
Vancomycin daily AUC0-24 and the ratio of creatinine change to baseline calculated by one- and two-point estimation. Each plot was stratified by AUC0-24 levels of (A, B) 500, (C, D) 600, and (E, F) 700 mg∙h/L. The P values represent Mann–Whitney U test results.
AUC0-24 = area under the curve over 24 hours.

When the threshold values of AUC
0-24 and trough concentration was evaluated by ROC curve for the prediction of AKI, AUC
0-24 threshold values in both one- and two-sample-based estimations showed similar results (524.26 vs. 563.45 mg*h/L; one- and two-sample-based estimations, respectively), and threshold values for the trough concentration showed mixed results (8.95 and 10.66 µg/mL; one- and two-sample-based estimation, respectively) (
Fig. 2). Among the AUC
0-24, trough threshold values and other risk factors evaluated by multivariate analysis, AUC
0-24 ≥ 563.45 mg*h/L in two-sample-based estimation (odds ratio [OR], 4.33; 95% confidence interval [CI], 1.18–15.87) and trough ≥ 8.95 µg/mL in one-sample-based estimation (OR, 8.47; 95% CI, 1.05–66.67) were significant predictors of AKI (
P = 0.027 and
P =0.045, respectively) (
Supplementary Table 1).
Fig. 2
ROC curve for estimation of (A, B) AUC0-24 and (C, D) trough cutoff values for AKI prediction. The AUC shown in the figure represents the area under the ROC curve.
ROC = receiver operating characteristic, AUC0-24 = area under the curve over 24 hours, AKI = acute kidney injury.


Nephrotoxicity is a widely known adverse side effect associated with the use of vancomycin.
111213 To date, collective data suggest a positive correlation between vancomycin trough level (15 and 20 µg/mL) and the risk of AKI, while the accurate association between AUC and the incidence of AKI remains an area of controversy.
1415161718 With the release of 2020 revised consensus guidelines suggesting a vancomycin AUC
0-24/MIC target of 400 to 600 mg*h/L, the appropriateness of the target AUC
0-24/MIC to predict and prevent AKI has become an area of special interest. While there were investigations to set the parameter threshold value for the prediction of AKI, most previous studies relied on a one-point sampling method to calculate the PK parameters, and comprehensive evaluation of different sampling methods and target parameters to predict the incidence of AKI has been absent.
151719
In the current study, we aimed to evaluate the newly suggested daily AUC
0-24/MIC target of 400-600 mg*h/L in context of the incidence of AKI. When the ROC curve was used to estimate the threshold values of AUC
0-24, the one-point method resulted in 524.26 mg*h/L, and the two-point method resulted in 563.45 mg*h/L. This cutoff is comparable to or lower than the previously suggested values between 563 and 1,300 mg*h/L.
15171820 Possible explanations for the variability observed between studies include the differences in the TDM methods used for AUC
0-24 calculation and patient-clinician disposition. One example of patient-clinician disposition is that if pharmacovigilance is more strictly applied in a study, patients with increasing serum creatinine levels are likely to experience termination of vancomycin dosing, resulting in overprediction of the AUC
0-24 cutoff value. In this study, only the first ever conducted vancomycin TDM data are included in the analysis to minimize selection-based bias.
When the ROC curve was used to estimate the threshold value of trough levels, the one-point method resulted in 8.95 µg/mL, and the two-point method resulted in 10.66 µg/mL. This cutoff is lower than the previously suggested therapeutic range of 15 to 20 µg/mL. Possible explanation for the observed discrepancies is that, some of the patients presented with AKI in this study showed comparatively low vancomycin trough concentration (up to 4 patients ranged 5–10 µg/mL, depending on TDM method), and may have contributed to under prediction of threshold value.
In the multivariate analysis, other possible risk factors that may have an effect on the incidence of AKI were investigated along with calculated vancomycin exposure threshold values. No association between risk factors and AKI was observed except AUC0-24 (563.45 mg*h/L) and trough (8.95 µg/mL) threshold values from two- and one-point-based prediction, respectively. This indicates that the calculated threshold values are independent predictors of AKI and may be used as the upper limit of the therapeutic target range.
Our data agreed well with the 2020 revised guidelines for the TDM of vancomycin. The calculated AUC0-24 value of 563.45 mg*h/L (two-point sampling method) compared well with the suggested upper limit of 600 mg*h/L. However, the trough threshold value calculated with a one-point sample was also associated with AKI (albeit inconsistent among sampling methods), and further investigation may be needed to investigate the optimal predictive methods. This result suggests that when a two-sample-based AUC0-24 calculation is not possible, using the trough cutoff value from the single-sampling method may be an alternative method for the TDM of vancomycin and the prediction of AKI. Establishment of a possible upper range of TDM targets allows optimal individualized dosing and dose monitoring of vancomycin to achieve a therapeutic range of drug exposure while minimizing the incidence of adverse events such as AKI.
Ethics statement
This study was approved by the Seoul National University Bundang Hospital Institutional Review Boards (SNUBH, IRBs), and informed consent for individual patients was not acquired [B-2101-663-107, January 2021].






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download