1. Malvezzi P, Rostaing L. 2015; The safety of calcineurin inhibitors for kidney-transplant patients. Expert Opin Drug Saf. 14:1531–46. DOI:
10.1517/14740338.2015.1083974. PMID:
26329325.
2. Gonwa T, Johnson C, Ahsan N, Alfrey EJ, Halloran P, Stegall M, et al. 2003; Randomized trial of tacrolimus+mycophenolate mofetil or azathioprine versus cyclosporine+mycophenolate mofetil after cadaveric kidney transplantation: results at three years. Transplantation. 75:2048–53. DOI:
10.1097/01.TP.0000069831.76067.22. PMID:
12829910.
3. Schuurman HJ, Cottens S, Fuchs S, Joergensen J, Meerloo T, Sedrani R, et al. 1997; SDZ RAD, a new rapamycin derivative: synergism with cyclosporine. Transplantation. 64:32–5. DOI:
10.1097/00007890-199707150-00007. PMID:
9233697.
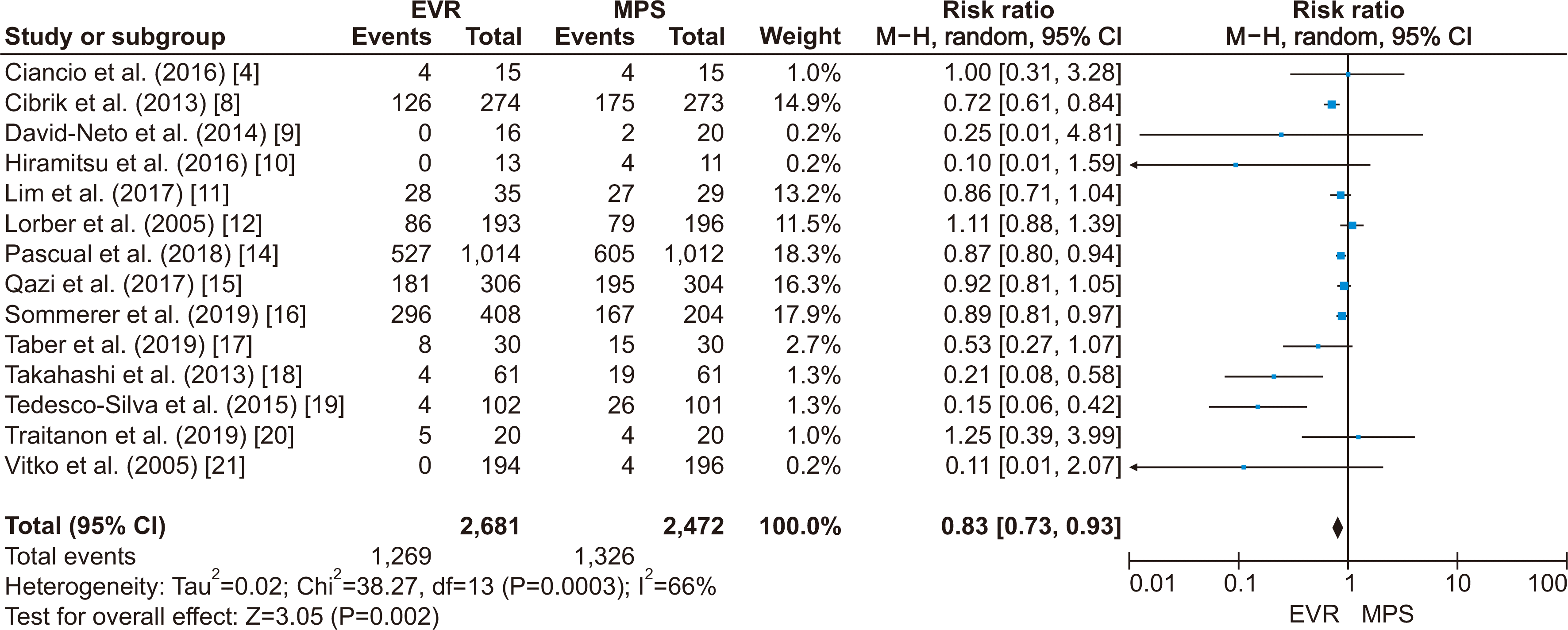
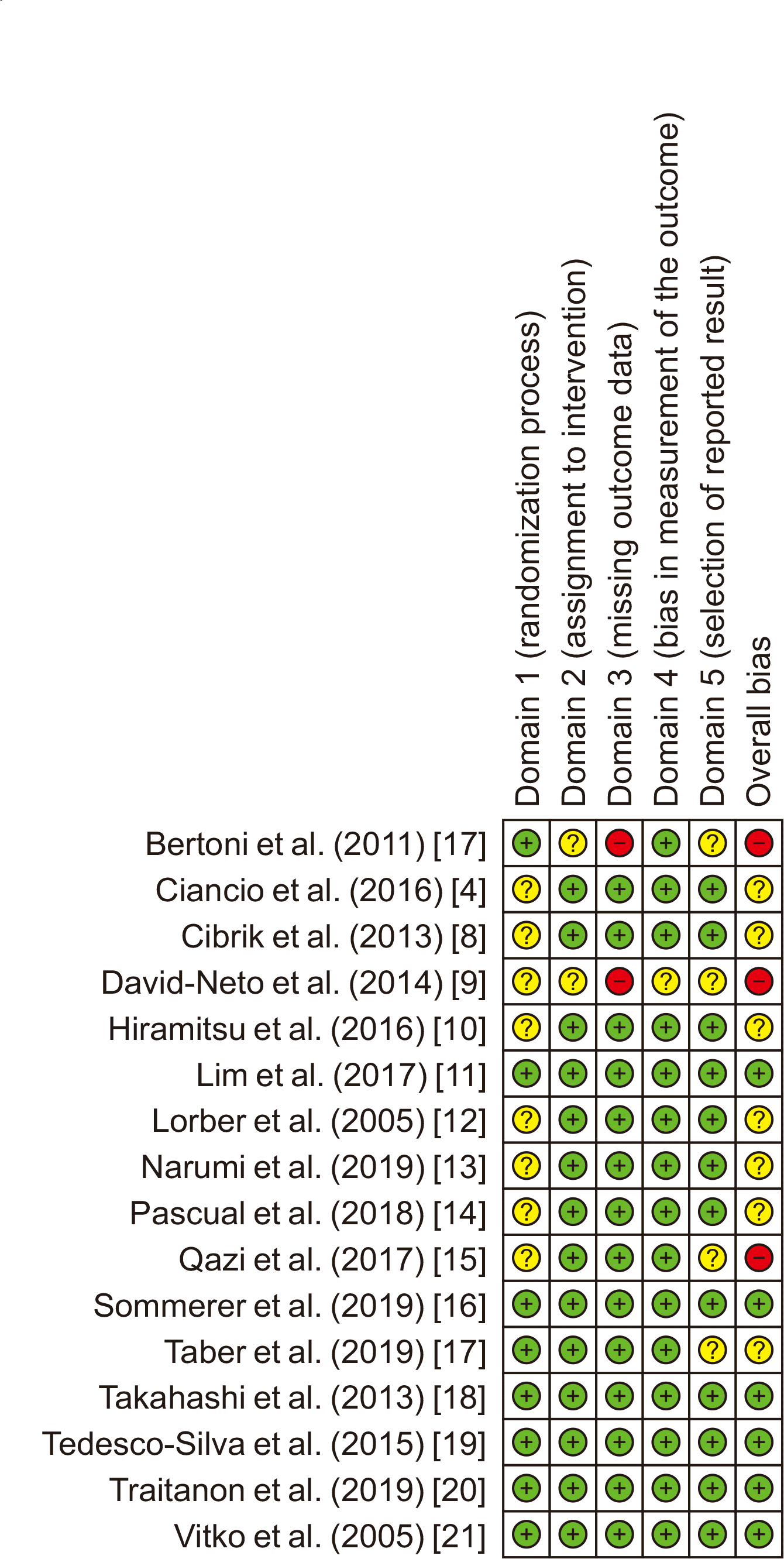
4. Ciancio G, Tryphonopoulos P, Gaynor JJ, Guerra G, Sageshima J, Roth D, et al. 2016; Pilot randomized trial of tacrolimus/everolimus vs tacrolimus/enteric-coated mycophenolate sodium in adult, primary kidney transplant recipients at a single center. Transplant Proc. 48:2006–10. DOI:
10.1016/j.transproceed.2016.03.048. PMID:
27569936.
5. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. 2021; The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 18:e1003583. DOI:
10.1371/journal.pmed.1003583. PMID:
33780438. PMCID:
PMC8007028.
6. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. 2019; RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ. 366:l4898. DOI:
10.1136/bmj.l4898. PMID:
31462531.
7. Bertoni E, Larti A, Rosso G, Zanazzi M, Di Maria L, Salvadori M. 2011; Good outcomes with cyclosporine very low exposure with everolimus high exposure in renal transplant patients. J Nephrol. 24:613–8. DOI:
10.5301/JN.2011.6247. PMID:
21240873.
8. Cibrik D, Silva HT Jr, Vathsala A, Lackova E, Cornu-Artis C, Walker RG, et al. 2013; Randomized trial of everolimus-facilitated calcineurin inhibitor minimization over 24 months in renal transplantation. Transplantation. 95:933–42. DOI:
10.1097/TP.0b013e3182848e03. PMID:
23422495.
9. David-Neto E, Galante N, Altona M, Paula F, Triboni A, Ramos F, et al. 2014; A randomized, prospective study comparing everolimus/low tacrolimus with regular tacrolimus/mps for the elderly renal transplant recipients. Transplantation. 98(Suppl 1):549. DOI:
10.1097/00007890-201407151-01841.
10. Hiramitsu T, Okada M, Futamura K, Yamamoto T, Tsujita M, Goto N, et al. 2016; 5-year follow-up of a randomized clinical study comparing everolimus plus reduced-dose cyclosporine with mycophenolate mofetil plus standard-dose cyclosporine in de novo kidney transplantation: retrospective single center assessment. Int Immunopharmacol. 39:192–8. DOI:
10.1016/j.intimp.2016.07.019. PMID:
27491025.
11. Lim WH, Russ GR, Wong G, Pilmore H, Kanellis J, Chadban SJ. 2017; The risk of cancer in kidney transplant recipients may be reduced in those maintained on everolimus and reduced cyclosporine. Kidney Int. 91:954–63. DOI:
10.1016/j.kint.2016.11.008. PMID:
28109543.
12. Lorber MI, Mulgaonkar S, Butt KM, Elkhammas E, Mendez R, Rajagopalan PR, et al. 2005; Everolimus versus mycophenolate mofetil in the prevention of rejection in de novo renal transplant recipients: a 3-year randomized, multicenter, phase III study. Transplantation. 80:244–52. DOI:
10.1097/01.TP.0000164352.65613.24. PMID:
16041270.
13. Narumi S, Watarai Y, Goto N, Hiramitsu T, Tsujita M, Okada M, et al. 2019; Everolimus-based immunosuppression possibly suppresses mean fluorescence intensity values of de novo donor-specific antibodies after primary kidney transplantation. Transplant Proc. 51:1378–81. DOI:
10.1016/j.transproceed.2019.03.019. PMID:
31056252.
14. Pascual J, Berger SP, Witzke O, Tedesco H, Mulgaonkar S, Qazi Y, et al. 2018; Everolimus with reduced calcineurin inhibitor exposure in renal transplantation. J Am Soc Nephrol. 29:1979–91. DOI:
10.1681/ASN.2018010009. PMID:
29752413. PMCID:
PMC6050928.
15. Qazi Y, Shaffer D, Kaplan B, Kim DY, Luan FL, Peddi VR, et al. 2017; Efficacy and safety of everolimus plus low-dose tacrolimus versus mycophenolate mofetil plus standard-dose tacrolimus in de novo renal transplant recipients: 12-month data. Am J Transplant. 17:1358–69. DOI:
10.1111/ajt.14090. PMID:
27775865.
16. Sommerer C, Suwelack B, Dragun D, Schenker P, Hauser IA, Witzke O, et al. 2019; An open-label, randomized trial indicates that everolimus with tacrolimus or cyclosporine is comparable to standard immunosuppression in de novo kidney transplant patients. Kidney Int. 96:231–44. DOI:
10.1016/j.kint.2019.01.041. PMID:
31027892.
17. Taber DJ, Chokkalingam A, Su Z, Self S, Miller D, Srinivas T. 2019; Randomized controlled trial assessing the impact of everolimus and low-exposure tacrolimus on graft outcomes in kidney transplant recipients. Clin Transplant. 33:e13679. DOI:
10.1111/ctr.13679.
18. Takahashi K, Uchida K, Yoshimura N, Takahara S, Teraoka S, Teshima R, et al. 2013; Efficacy and safety of concentration-controlled everolimus with reduced-dose cyclosporine in Japanese de novo renal transplant patients: 12-month results. Transplant Res. 2:14. DOI:
10.1186/2047-1440-2-14. PMID:
23866828. PMCID:
PMC3718642.

19. Tedesco-Silva H, Felipe C, Ferreira A, Cristelli M, Oliveira N, Sandes-Freitas T, et al. 2015; Reduced incidence of cytomegalovirus infection in kidney transplant recipients receiving everolimus and reduced tacrolimus doses. Am J Transplant. 15:2655–64. DOI:
10.1111/ajt.13327. PMID:
25988935.

20. Traitanon O, Mathew JM, Shetty A, Bontha SV, Maluf DG, El Kassis Y, et al. 2019; Mechanistic analyses in kidney transplant recipients prospectively randomized to two steroid free regimen-low dose tacrolimus with everolimus versus standard dose tacrolimus with mycophenolate mofetil. PLoS One. 14:e0216300. DOI:
10.1371/journal.pone.0216300. PMID:
31136582. PMCID:
PMC6538151.

21. Vítko S, Margreiter R, Weimar W, Dantal J, Kuypers D, Winkler M, et al. 2005; Three-year efficacy and safety results from a study of everolimus versus mycophenolate mofetil in de novo renal transplant patients. Am J Transplant. 5:2521–30. DOI:
10.1111/j.1600-6143.2005.01063.x. PMID:
16162203.

22. Danovitch GM. Handbook of kidney transplantation. Wolters Kluwer;2017.
23. Xie X, Jiang Y, Lai X, Xiang S, Shou Z, Chen J. 2015; mTOR inhibitor versus mycophenolic acid as the primary immunosuppression regime combined with calcineurin inhibitor for kidney transplant recipients: a meta-analysis. BMC Nephrol. 16:91. DOI:
10.1186/s12882-015-0078-5. PMID:
26126806. PMCID:
PMC4486141.
24. Liu J, Liu D, Li J, Zhu L, Zhang C, Lei K, et al. 2017; Efficacy and safety of everolimus for maintenance immunosuppression of kidney transplantation: a meta-analysis of randomized controlled trials. PLoS One. 12:e0170246. DOI:
10.1371/journal.pone.0170246. PMID:
28107397. PMCID:
PMC5249216.

25. Gao L, Xu F, Cheng H, Liu J. 2018; Comparison of sirolimus combined with tacrolimus and mycophenolate mofetil combined with tacrolimus in kidney transplantation recipients: a meta-analysis. Transplant Proc. 50:3306–13. DOI:
10.1016/j.transproceed.2018.08.056. PMID:
30577200.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download