1. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019; 139:e56–528. PMID:
30700139.
2. Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013; 88:720–755. PMID:
23790560.
3. Molinari C, Morsanuto V, Polli S, Uberti F. Cooperative effects of Q10, vitamin D
3, and L-arginine on cardiac and endothelial cells. J Vasc Res. 2018; 55:47–60. PMID:
29301117.
4. Cardoso FE, Dos Santos LD, Tenório AP, Lopes MR, Barbosa RH. Supplementation with vitamin D and its analogs for treatment of endothelial dysfunction and cardiovascular disease. J Vasc Bras. 2020; 19:e20190150. PMID:
34178073.
5. Kastrup J. Can YKL-40 be a new inflammatory biomarker in cardiovascular disease? Immunobiology. 2012; 217:483–491. PMID:
21601307.
6. Kim HM, Lee BW, Song YM, et al. Potential association between coronary artery disease and the inflammatory biomarker YKL-40 in asymptomatic patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2012; 11:84. PMID:
22809439.
7. Deng X, Zhao L, Guo C, et al. Higher serum asprosin level is associated with urinary albumin excretion and renal function in type 2 diabetes. Diabetes Metab Syndr Obes. 2020; 13:4341–4351. PMID:
33223841.
8. Kjaergaard AD, Johansen JS, Bojesen SE, Nordestgaard BG. Role of inflammatory marker YKL-40 in the diagnosis, prognosis and cause of cardiovascular and liver diseases. Crit Rev Clin Lab Sci. 2016; 53:396–408. PMID:
27187575.
9. Faridvand Y, Bagherpour-Hassanlouei N, Nozari S, et al. 1, 25-Dihydroxyvitamin D3 activates apelin/APJ system and inhibits the production of adhesion molecules and inflammatory mediators in LPS-activated RAW264.7 cells. Pharmacol Rep. 2019; 71:811–817. PMID:
31377563.
10. Liu L, Shi Z, Ji X, et al. Adipokines, adiposity, and atherosclerosis. Cell Mol Life Sci. 2022; 79:272. PMID:
35503385.
11. Beveridge LA, Khan F, Struthers AD, et al. Effect of vitamin D supplementation on markers of vascular function: a systematic review and individual participant meta-analysis. J Am Heart Assoc. 2018; 7:e008273. PMID:
29848497.
12. Abulmeaty MM, Almajwal AM, Alam I, et al. Relationship of vitamin D-deficient diet and irisin, and their impact on energy homeostasis in rats. Front Physiol. 2020; 11:25. PMID:
32082189.
13. Balci T, Kocabas R, Cuce G, Akoz M. Inhibition of fatty acid binding protein 4 in obese male mice adversely affects reproductive parameters. J Reprod Infertil. 2021; 22:16–22. PMID:
33680881.
14. Yin K, You Y, Swier V, et al. Vitamin D protects against atherosclerosis via regulation of cholesterol efflux and macrophage polarization in hypercholesterolemic swine. Arterioscler Thromb Vasc Biol. 2015; 35:2432–2442. PMID:
26381871.
15. Lee SH, Kim DH, Youn YN, et al. Effect of rosuvastatin on bovine pericardial aortic tissue valve calcification in a rat subdermal implantation model. Korean Circ J. 2017; 47:401–408. PMID:
28567091.
16. Kocabas R, Akoz M. The effects of vitamin D supplementation on healthy and hypercholesterolemic rabbits on levels of OSI and paraoxonase. Turk Biyokim Derg. 2018; 43:549–556.
17. Wang JH, Keisala T, Solakivi T, Minasyan A, Kalueff AV, Tuohimaa P. Serum cholesterol and expression of ApoAI, LXRbeta and SREBP2 in vitamin D receptor knock-out mice. J Steroid Biochem Mol Biol. 2009; 113:222–226. PMID:
19429425.
18. Soave O, Brand CD. Coprophagy in animals: a review. Cornell Vet. 1991; 81:357–364. PMID:
1954740.
19. Wolf ST, Dillon GA, Alexander LM, Jablonski NG, Kenney WL. Skin pigmentation is negatively associated with circulating vitamin D concentration and cutaneous microvascular endothelial function. Am J Physiol Heart Circ Physiol. 2022; 323:490–498. PMID:
35930446.
20. Zhou Q, Han X, Li R, et al. Anti-atherosclerosis of oligomeric proanthocyanidins from
Rhodiola rosea on rat model via hypolipemic, antioxidant, anti-inflammatory activities together with regulation of endothelial function. Phytomedicine. 2018; 51:171–180. PMID:
30466614.
21. Aköz M, Kocabaş R, Topçu C, Gürbilek M. The effects of trans-9 18:1 octadecenoic acid isomer on levels of sICAM-1, svICAM-1 and IGF-1. Turk J Biochem. 2011; 36:1–5.
22. Omidian M, Mahmoudi M, Javanbakht MH, et al. Effects of vitamin D supplementation on circulatory YKL-40 and MCP-1 biomarkers associated with vascular diabetic complications: a randomized, placebo-controlled, double-blind clinical trial. Diabetes Metab Syndr. 2019; 13:2873–2877. PMID:
31425951.
23. Malyszko J, Koc-Zorawska E, Malyszko J. YKL-40, a marker of cardiovascular disease and endothelial dysfunction, in kidney transplant recipients. Transplantation Proceedings. Amsterdam: Elsevier Inc.;2014. p. 2651–2653.
24. Tong X, Wang D, Liu S, et al. The YKL-40 protein is a potential biomarker for COPD: a meta-analysis and systematic review. Int J Chron Obstruct Pulmon Dis. 2018; 13:409–418. PMID:
29430175.
25. Deng Y, Li G, Chang D, Su X. YKL-40 as a novel biomarker in cardio-metabolic disorders and inflammatory diseases. Clin Chim Acta. 2020; 511:40–46. PMID:
33002471.
26. Naeini AE, Moeinzadeh F, Vahdat S, Ahmadi A, Hedayati ZP, Shahzeidi S. The effect of Vitamin D administration on intracellular adhesion molecule-1 and vascular cell adhesion molecule-1 levels in hemodialysis patients: a placebo-controlled, double-blinded clinical trial. J Res Pharm Pract. 2017; 6:16–20. PMID:
28331861.
27. Askin L, Tibilli H, Tanriverdi O, Turkmen S. The relationship between coronary artery disease and SIRT1 protein. North Clin Istanb. 2020; 7:631–635. PMID:
33381707.

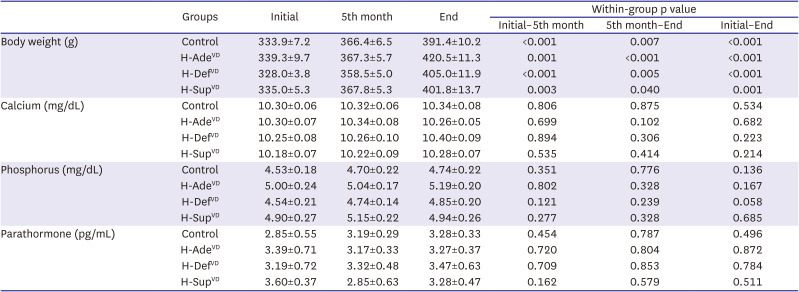
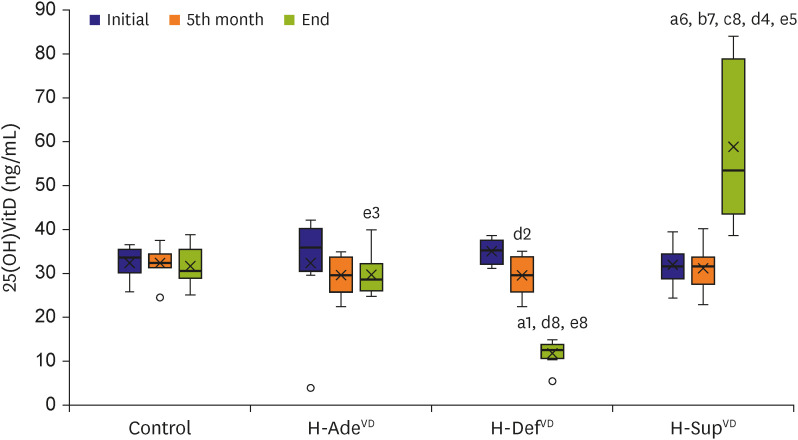
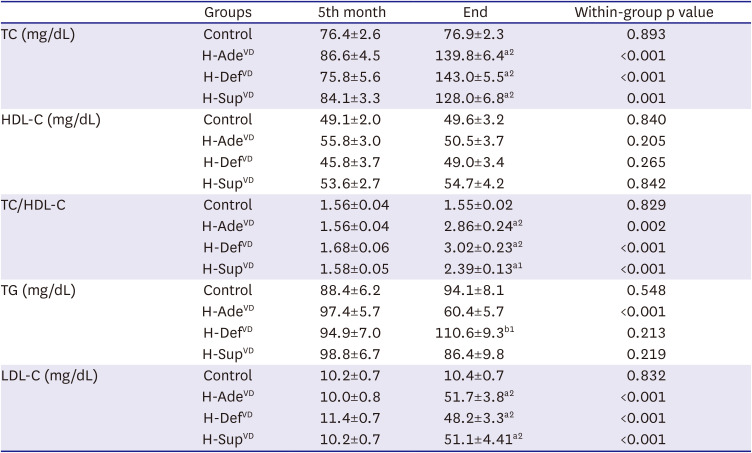
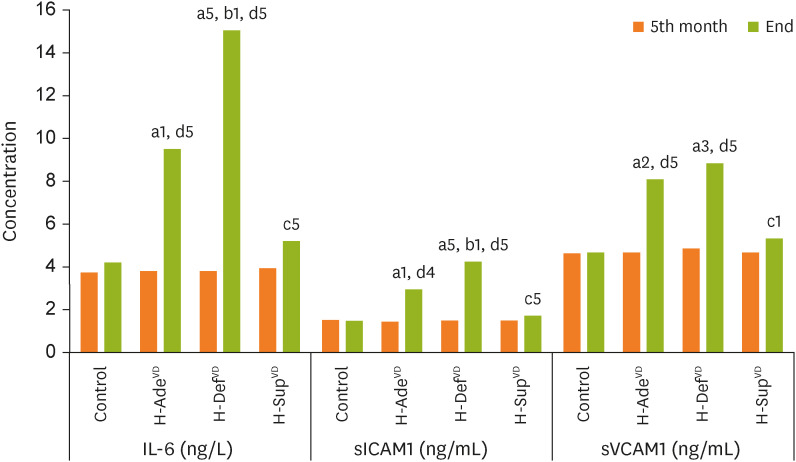




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download