Abstract
Lactase non-persistence (LNP), one of the causes of lactose intolerance, is related to lactase gene associated single nucleotide polymorphisms (SNPs). Since the frequency of LNP varies by ethnic group and country, the research to reveal the presence or absence of LNP for specific people has been conducted worldwide. However, in East Asia, the study of lactase gene associated SNPs have not been sufficiently examined so far using ancient human specimens from archaeological sites. In our study of Joseon period human remains (n=14), we successfully revealed genetic information of lactase gene associated SNPs (rs1679771596, rs41525747, rs4988236, rs4988235, rs41380347, rs869051967, rs145946881 and rs182549), further confirming that as for eight SNPs, the pre-modern Korean people had a lactase non-persistent genotype. Our report contributes to the establishment of LNP associated SNP analysis technique that can be useful in forthcoming studies on human bones and mummy samples from East Asian archaeological sites.
Lactose is a disaccharide of glucose and galactose, which accounts for most of the carbohydrates included in milk. Milk is a nutritionally ideal food, but people often suffer from digestive difficulties because the lactose of milk is not digested well but fermented by intestinal microorganisms [1, 2]. This symptom is called lactose intolerance [3].
Bayless and Rosensweig [4] found that the rates of lactose intolerance between African Americans (70%) and populations of a recent European descent (5%) differed significantly and thought that lactase activity would be genetically regulated. Later, Enattah et al. [5] also argued that regulatory genes present on human chromosome 2 are associated with lactase non-persistence (LNP), which causes lactose intolerance [5, 6]. Lactase gene is about 50 kb in size, and the genotype of single nucleotide polymorphism (SNP, LCT-13910 or rs4988235) determines whether the subject has LNP or not in people of a recent European descent. In brief, the genotype of SNP for LNP is C/C; lactase persistence (LP) is T/T; and the activity of lactase can be intermediate as C/T [5].
Currently, it is possible to estimate the global distribution of LNP through the study of its frequency worldwide. That is, Ingram et al. [7] estimated that about 70% of the world’s population has the genotype of LCT-13910 as C/C, which tends to be LNP. Considering the global frequency, lactose intolerance rate caused by LNP looks varied by ethnic group and region worldwide. By country, lactose intolerance due to LNP was found to be almost 100% in Asia, in South America at 50%–90%, and in American Indians at 80%, but in Europe and populations of a recent European descent at 10%–20% [7-9]. In Korea, Enattah et al. [10] also confirmed that the LNP frequency in the country was 100% by study of rs4988235 on 46 Koreans.
Recently, research trends on LNP are expanding beyond simply confirming the SNP frequency of modern people. Genetic analysis has led to differences in frequency, expanding to geographical distribution of lactose intolerance in history, thus answering the quest of human migration based on the genetic evidence [11-14]. Nevertheless, in East Asian countries including South Korea, no technical foundation has been made because very few research on LNP has been conducted so far on the archaeologically obtained human remains in the region.
The genetic analysis was conducted on fourteen human bones and mummies found in a total of nine Joseon period graves. Samples were attained at archaeological sites of Eunpyeong (Seoul), Sinnae (Seoul), Yongin (Gyeonggi-Do), Uijeongbu (Gyeonggi-Do), Sapgyo (Chungcheongnam-Do), Wonju (Gangwon-Do), Waegwan (Gyeongsangbuk-Do), Hadong (Gyeongsangnam-Do). The detailed information is summarized in Table 1. In order to secure the authenticity of genetic analysis, the guidelines of Hofreiter et al. [15] and Willerslev and Cooper [16] were respected in our study. In brief, participants wore sterile gowns, headcaps, masks, and two layers of gloves during aDNA work. Our aDNA facility were spatially separated from the space of modern DNA work, equipped with a laminar flow clean bench, isolated ventilation, and UV irradiation. The aDNA facility is irradiated with UV light for 2 hours before and after use, and all experimental tools were used after cleaning with bleaching solution containing 0.5% sodium hypochlorite. The entire process, from sampling at the excavation site to genetic analysis in the laboratory, was conducted by researchers wearing sterilized gowns with sterilized tools.
This study was conducted after obtaining a review exemption from the Institutional Review Board of Seoul National University Hospital (IRB No. 2017-001) and Eulji University (IRB No. EU22-40).
The analyzed samples are mummified brains or femora. The method of extracting DNA from them is already described in Kim et al. [17] and Oh et al. [18]. In brief, the mummified brain tissue was used for DNA extraction after removing surface with a sterilized knife and irradiating with 254 nm of ultraviolet light for 20 minutes. The contamination of femur surface was also removed by a sterilized drill, and then washed and dehydrated by sequentially applying 0.5% sodium hypochlorite solution, distilled water, 70% and 100% ethanol. The bone powder was made after dried under UV irradiation inside the clean bench. For DNA extraction, bone (0.5–1 g) and brain tissue (0.2–0.3 g) were added to lysis buffer (EDTA 50 mM, pH 8.0; 1 mg/ml of proteinase K; SDS 1%) and reacted at 56°C for 24 hours. Then, the reaction solution was treated with phenol: chloroform: isoamyl alcohol (25:24:1) (Sigma, St Louis, MO, USA), chloroform: isoamyl alcohol (24:1) (Sigma), and the QIAquick PCR purification Kit (Qiagen, Hilden, Germany). The amount of purified DNA was measured by NanoDrop ND-1000 spectrophotometry (Thermo Fisher Scientific, Wilmington, DE, USA). DNA extraction was repeated twice per sample.
To remove uracil remained in extracted DNA, 20 µl of DNA was reacted at 37°C for 3 hours using 4 units of USER enzyme (New England Biolabs, Ipswich, MA, USA) and then purified using MinElute PCR Purification Kit (Qiagen). Uracil-removed aDNA (40 ng) was mixed with 1X AmpliTaq Gold 360 Master Mix (Life Technologies, Camarillo, CA, USA) and 20 pmol of PCR primers (Genotech, Daejeon, Korea). PCR conditions and each primer set information are summarized in Table 2. Briefly, two primer sets are designed to amplify eight SNPs, which known to be associated with the expression of the lactase gene [19, 20]. Of them, the amplicon of Lac-1 primer set (233 bp) is designed to include a total of seven SNPs, including rs1679771596 (LCT-13906), rs41525747 (LCT-13907), rs4988236 (LCT-13908), rs4988235 (LCT-13910), rs41380347 (LCT-13915), rs869051967 (LCT-14009), and rs145946881 (LCT-14010). The amplicon of Lac-2 primer set (147 bp) is also designed to contain one SNP (rs182549 or LCT-22018). PCR was conducted twice with different extractions of each sample.
The electrophoresis of PCR products was done on agarose gel (2.0%–2.5%), stained with ethidium bromide, and recorded by UV transilluminator and CCD camera. The amplification product was extracted using a Qiagen gel extraction kit (Qiagen), and sequenced at Macrogen Inc. (Seoul, Korea). The sequence analysis results were confirmed using the MEGA X program (https://www.megasoftware.net/) [21].
In PCR amplification using Lac-1 and Lac-2 primer sets and electrophoresis, specific amplicons of rs1679771596 (LCT-13906), rs41525747 (LCT-13907), rs4988236 (LCT-13908), rs4988235 (LCT-13910), rs41380347 (LCT-13915), rs869051967 (LCT-14009), rs145946881 (LCT-14010) and rs182549 (LCT-22018) could be successfully obtained (Fig. 1A, B). Next, DNA sequence analysis was conducted for each SNPs. The sequencing results confirmed that the amplicons include those lactase SNPs (Fig. 1C). In our SNP analyses of 14 Joseon period individuals, the results were obtained successfully (Supplementary Figs. 1 and 2). We found that Joseon individuals had the same genotype for all eight SNPs: allele A for rs1679771596 (LCT-13906T), G for rs4152747 (LCT-13907C), G for rs4988236 (LCT-13908C), G for rs4988235 (LCT-13910C), A for rs41380347 (LCT-13915T), A for rs869051967 (LCT-14009T), C for rs145946881 (LCT-14010G), and C for rs182549 (LCT-22018G). By these results, we confirmed that every Joseon people of this study had LNP genotypes for the eight SNPs (Table 3).
To confirm whether the results of this experiment are contaminated by modern DNA from researchers, mitochondrial DNA hypervariable region was amplified and sequenced for each ancient sample as well as the researchers (Supplementary Fig. 3). We confirmed that the haplotypes of researchers and Joseon period individuals were all different from each other. This means that there was no contamination of samples by researchers’ modern DNA (Table 4).
The frequency analysis of LNP through genetic analysis has been recently conducted around the world; therefore, much information has been accumulated so far. Besides the SNPs of modern people, human specimens collected at archaeological sites are also attracting anthropologists’ attention because the results of the study are important in terms of the emergence of lactose intolerance in mankind history.
As for the research of SNPs using ancient specimens, Burger et al. [22] attained LP-associated genotypes from Neolithic and Mesolithic European human remains. Nagy et al. [23] also revealed the prevalence of LNP related SNP (LCT-13910C/T) using ancient bone samples from Carpathian basin. Plantinga et al. [24] investigated LNP of Late Neolithic people (Basque Country) through aDNA analysis. Krüttli et al. [25] extracted DNA from medieval individuals of Dalheim, Germany, to see genotypes of LCT-13910C/T SNP. Płoszaj et al. [26] reported a SNP (LCT-13910) of Polish individuals buried at the medieval cemetery in Pień, central Poland. Mnich et al. [27] analyzed LCT-13910 genotypes of medieval skeletal individuals found at South-Eastern Poland. Keller et al. [28] conducted a genetic analysis of the 5,000-year-old mummy (‘Iceman’) found in Tyrol (Italy), confirming that the mummy had a problem with lactose intake by LNP during its lifetime. This means that LP was rare among Europeans even in the Neolithic Age; and after then, its LP increased and expanded to the entire European population [28]. This estimation was validated again by the study of Saag et al. [14] on human individuals who lived in Europe between 6,000 BC and 1500 AD.
LNP analysis using SNP has been very meaningful in that it can academically prove historical changes in LP frequency and further infer human migration from them [12]. However, it is also true that the reports so far have unsatisfactory aspects as well. First, aDNA studies of LNP related SNPs have been mostly conducted on European people, so information about ancient people in other continents is insufficient. Especially in the case of East Asia, there is little information on aDNA of LNP-related SNPs. Jeong et al. [13, 29] revealed if LP allele (LCT-13910T) was present among Bronze Age to Medieval period Mongolian. And Ning et al. [30] showed that ancient people (7500-1700 BP) from northern China were genetically lactose intolerant. Other than these, no related aDNA research has been reported from other East Asian countries. Since there is a need to conduct aDNA research related to LNP associated SNPs in Asia, our study can be meaningful in terms of establishing technical foundation for future SNP analysis in this region.
Notes
References
1. Lomer MC, Parkes GC, Sanderson JD. 2008; Review article: lactose intolerance in clinical practice--myths and realities. Aliment Pharmacol Ther. 27:93–103. DOI: 10.1111/j.1365-2036.2007.03557.x. PMID: 17956597.

2. Levitt M, Wilt T, Shaukat A. 2013; Clinical implications of lactose malabsorption versus lactose intolerance. J Clin Gastroenterol. 47:471–80. DOI: 10.1097/MCG.0b013e3182889f0f. PMID: 23632346.

3. Jarrett EC, Holman GH. 1966; Lactose absorption in the premature infant. Arch Dis Child. 41:525–7. DOI: 10.1136/adc.41.219.525. PMID: 5957726. PMCID: PMC2019603.

4. Bayless TM, Rosensweig NS. 1966; A racial difference in incidence of lactase deficiency. A survey of milk intolerance and lactase deficiency in healthy adult males. JAMA. 197:968–72. DOI: 10.1001/jama.1966.03110120074017. PMID: 5953213.

5. Enattah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, Järvelä I. 2002; Identification of a variant associated with adult-type hypolactasia. Nat Genet. 30:233–7. DOI: 10.1038/ng826. PMID: 11788828.

6. Järvelä IE. 2005; Molecular diagnosis of adult-type hypolactasia (lactase non-persistence). Scand J Clin Lab Invest. 65:535–9. DOI: 10.1080/00365510500208316. PMID: 16271984.

7. Ingram CJ, Mulcare CA, Itan Y, Thomas MG, Swallow DM. 2009; Lactose digestion and the evolutionary genetics of lactase persistence. Hum Genet. 124:579–91. DOI: 10.1007/s00439-008-0593-6. PMID: 19034520.

8. Swallow DM. 2003; Genetics of lactase persistence and lactose intolerance. Annu Rev Genet. 37:197–219. DOI: 10.1146/annurev.genet.37.110801.143820. PMID: 14616060.

9. Raz M, Sharon Y, Yerushalmi B, Birk R. 2013; Frequency of LCT-13910C/T and LCT-22018G/A single nucleotide polymorphisms associated with adult-type hypolactasia/lactase persistence among Israelis of different ethnic groups. Gene. 519:67–70. DOI: 10.1016/j.gene.2013.01.049. PMID: 23415628.

10. Enattah NS, Trudeau A, Pimenoff V, Maiuri L, Auricchio S, Greco L, Rossi M, Lentze M, Seo JK, Rahgozar S, Khalil I, Alifrangis M, Natah S, Groop L, Shaat N, Kozlov A, Verschubskaya G, Comas D, Bulayeva K, Mehdi SQ, Terwilliger JD, Sahi T, Savilahti E, Perola M, Sajantila A, Järvelä I, Peltonen L. 2007; Evidence of still-ongoing convergence evolution of the lactase persistence T-13910 alleles in humans. Am J Hum Genet. 81:615–25. DOI: 10.1086/520705. PMID: 17701907. PMCID: PMC1950831.

11. Sverrisdóttir OÓ, Timpson A, Toombs J, Lecoeur C, Froguel P, Carretero JM, Arsuaga Ferreras JL, Götherström A, Thomas MG. 2014; Direct estimates of natural selection in Iberia indicate calcium absorption was not the only driver of lactase persistence in Europe. Mol Biol Evol. 31:975–83. DOI: 10.1093/molbev/msu049. PMID: 24448642.

12. Allentoft ME, Sikora M, Sjögren KG, Rasmussen S, Rasmussen M, Stenderup J, Damgaard PB, Schroeder H, Ahlström T, Vinner L, Malaspinas AS, Margaryan A, Higham T, Chivall D, Lynnerup N, Harvig L, Baron J, Della Casa P, Dąbrowski P, Duffy PR, Ebel AV, Epimakhov A, Frei K, Furmanek M, Gralak T, Gromov A, Gronkiewicz S, Grupe G, Hajdu T, Jarysz R, Khartanovich V, Khokhlov A, Kiss V, Kolář J, Kriiska A, Lasak I, Longhi C, McGlynn G, Merkevicius A, Merkyte I, Metspalu M, Mkrtchyan R, Moiseyev V, Paja L, Pálfi G, Pokutta D, Pospieszny Ł, Price TD, Saag L, Sablin M, Shishlina N, Smrčka V, Soenov VI, Szeverényi V, Tóth G, Trifanova SV, Varul L, Vicze M, Yepiskoposyan L, Zhitenev V, Orlando L, Sicheritz-Pontén T, Brunak S, Nielsen R, Kristiansen K, Willerslev E. 2015; Population genomics of Bronze Age Eurasia. Nature. 522:167–72. DOI: 10.1038/nature14507. PMID: 26062507.

13. Jeong C, Wilkin S, Amgalantugs T, Bouwman AS, Taylor WTT, Hagan RW, Bromage S, Tsolmon S, Trachsel C, Grossmann J, Littleton J, Makarewicz CA, Krigbaum J, Burri M, Scott A, Davaasambuu G, Wright J, Irmer F, Myagmar E, Boivin N, Robbeets M, Rühli FJ, Krause J, Frohlich B, Hendy J, Warinner C. 2018; Bronze Age population dynamics and the rise of dairy pastoralism on the eastern Eurasian steppe. Proc Natl Acad Sci U S A. 115:E11248–55. DOI: 10.1073/pnas.1813608115. PMID: 30397125. PMCID: PMC6275519.

14. Saag L, Laneman M, Varul L, Malve M, Valk H, Razzak MA, Shirobokov IG, Khartanovich VI, Mikhaylova ER, Kushniarevich A, Scheib CL, Solnik A, Reisberg T, Parik J, Saag L, Metspalu E, Rootsi S, Montinaro F, Remm M, Mägi R, D'Atanasio E, Crema ER, Díez-Del-Molino D, Thomas MG, Kriiska A, Kivisild T, Villems R, Lang V, Metspalu M, Tambets K. 2019; The arrival of Siberian ancestry connecting the eastern Baltic to Uralic speakers further east. Curr Biol. 29:1701–11.e16. DOI: 10.1016/j.cub.2019.04.026. PMID: 31080083. PMCID: PMC6544527.

15. Hofreiter M, Serre D, Poinar HN, Kuch M, Pääbo S. 2001; Ancient DNA. Nat Rev Genet. 2:353–9. DOI: 10.1038/35072071. PMID: 11331901.

16. Willerslev E, Cooper A. 2005; Ancient DNA. Proc Biol Sci. 272:3–16. DOI: 10.1098/rspb.2004.2813. PMID: 15875564. PMCID: PMC1634942.
17. Kim YS, Oh CS, Lee SJ, Park JB, Kim MJ, Shin DH. 2011; Sex determination of Joseon people skeletons based on anatomical, cultural and molecular biological clues. Ann Anat. 193:539–43. DOI: 10.1016/j.aanat.2011.07.002. PMID: 21889322.

18. Oh CS, Hong JH, Shin DH. 2019; Mitochondrial DNA analysis of the human skeletons from Goryeo dynasty graves discovered at Youngwol, Gangwon-do. Anat Biol Anthropol. 32:61–7. DOI: 10.11637/aba.2019.32.2.61.

19. Kato K, Ishida S, Tanaka M, Mitsuyama E, Xiao JZ, Odamaki T. 2018; Association between functional lactase variants and a high abundance of Bifidobacterium in the gut of healthy Japanese people. PLoS One. 13:e0206189. DOI: 10.1371/journal.pone.0206189. PMID: 30339693. PMCID: PMC6195297.

20. Peng MS, He JD, Zhu CL, Wu SF, Jin JQ, Zhang YP. 2012; Lactase persistence may have an independent origin in Tibetan populations from Tibet, China. J Hum Genet. 57:394–7. DOI: 10.1038/jhg.2012.41. PMID: 22572735.

21. Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018; MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35:1547–9. DOI: 10.1093/molbev/msy096. PMID: 29722887. PMCID: PMC5967553.

22. Burger J, Kirchner M, Bramanti B, Haak W, Thomas MG. 2007; Absence of the lactase-persistence-associated allele in early Neolithic Europeans. Proc Natl Acad Sci U S A. 104:3736–41. DOI: 10.1073/pnas.0607187104. PMID: 17360422. PMCID: PMC1820653.

23. Nagy D, Tömöry G, Csányi B, Bogácsi-Szabó E, Czibula Á, Priskin K, Bede O, Bartosiewicz L, Downes CS, Raskó I. 2011; Comparison of lactase persistence polymorphism in ancient and present-day Hungarian populations. Am J Phys Anthropol. 145:262–9. DOI: 10.1002/ajpa.21490. PMID: 21365615.

24. Plantinga TS, Alonso S, Izagirre N, Hervella M, Fregel R, van der Meer JW, Netea MG, de la Rúa C. 2012; Low prevalence of lactase persistence in Neolithic South-West Europe. Eur J Hum Genet. 20:778–82. Erratum in: Eur J Hum Genet 2012;20:810. DOI: 10.1038/ejhg.2011.254. PMID: 22234158. PMCID: PMC3376259.

25. Krüttli A, Bouwman A, Akgül G, Della Casa P, Rühli F, Warinner C. 2014; Ancient DNA analysis reveals high frequency of European lactase persistence allele (T-13910) in medieval central europe. PLoS One. 9:e86251. DOI: 10.1371/journal.pone.0086251. PMID: 24465990. PMCID: PMC3900515. PMID: d475bd5f2ed640f3b8ccbe524cd52e65.

26. Płoszaj T, Jędrychowska-Dańska K, Zamerska A, Drozd-Lipińska A, Poliński D, Janowski A, Witas H. 2017; Ancient DNA analysis might suggest external origin of individuals from chamber graves placed in medieval cemetery in Pień, Central Poland. Anthropol Anz. 74:319–37. DOI: 10.1127/anthranz/2017/0727. PMID: 28799621.

27. Mnich B, Spinek AE, Chyleński M, Sommerfeld A, Dabert M, Juras A, Szostek K. 2018; Analysis of LCT-13910 genotypes and bone mineral density in ancient skeletal materials. PLoS One. 13:e0194966. Erratum in: PLoS One 2020;15:e0236908. DOI: 10.1371/journal.pone.0236908. PMID: 32702066. PMCID: PMC7377453. PMID: c071ffd7bba3433c8f2f9e7f45053b97.

28. Keller A, Graefen A, Ball M, Matzas M, Boisguerin V, Maixner F, Leidinger P, Backes C, Khairat R, Forster M, Stade B, Franke A, Mayer J, Spangler J, McLaughlin S, Shah M, Lee C, Harkins TT, Sartori A, Moreno-Estrada A, Henn B, Sikora M, Semino O, Chiaroni J, Rootsi S, Myres NM, Cabrera VM, Underhill PA, Bustamante CD, Vigl EE, Samadelli M, Cipollini G, Haas J, Katus H, O'Connor BD, Carlson MR, Meder B, Blin N, Meese E, Pusch CM, Zink A. 2012; New insights into the Tyrolean Iceman's origin and phenotype as inferred by whole-genome sequencing. Nat Commun. 3:698. DOI: 10.1038/ncomms1701. PMID: 22426219.

29. Jeong C, Wang K, Wilkin S, Taylor WTT, Miller BK, Bemmann JH, Stahl R, Chiovelli C, Knolle F, Ulziibayar S, Khatanbaatar D, Erdenebaatar D, Erdenebat U, Ochir A, Ankhsanaa G, Vanchigdash C, Ochir B, Munkhbayar C, Tumen D, Kovalev A, Kradin N, Bazarov BA, Miyagashev DA, Konovalov PB, Zhambaltarova E, Miller AV, Haak W, Schiffels S, Krause J, Boivin N, Erdene M, Hendy J, Warinner C. 2020; A dynamic 6,000-year genetic history of Eurasia's Eastern Steppe. Cell. 183:890–904.e29. DOI: 10.1016/j.cell.2020.10.015. PMID: 33157037. PMCID: PMC7664836.

30. Ning C, Li T, Wang K, Zhang F, Li T, Wu X, Gao S, Zhang Q, Zhang H, Hudson MJ, Dong G, Wu S, Fang Y, Liu C, Feng C, Li W, Han T, Li R, Wei J, Zhu Y, Zhou Y, Wang CC, Fan S, Xiong Z, Sun Z, Ye M, Sun L, Wu X, Liang F, Cao Y, Wei X, Zhu H, Zhou H, Krause J, Robbeets M, Jeong C, Cui Y. 2020; Ancient genomes from northern China suggest links between subsistence changes and human migration. Nat Commun. 11:2700. DOI: 10.1038/s41467-020-16557-2. PMID: 32483115. PMCID: PMC7264253. PMID: 952b238525f04d65a6fd937f71e31d06.

31. Holland MM, Huffine EF. 2001; Molecular analysis of the human mitochondrial DNA control region for forensic identity testing. Curr Protoc Hum Genet. Chapter 14:Unit 14.7. DOI: 10.1002/0471142905.hg1407s74. PMID: 22786611.

Fig. 1
A brief example of the genetic analysis procedure. (A) PCR result for Lac-1 primer set. (B) PCR result for Lac-2 primer set. (C) Analysis of electropherogram results of rs1679771596 (LCT-13906), rs41525747 (LCT-13907), rs4988236 (LCT-13908), rs4988235 (LCT-13910), rs41380347 (LCT-13915), rs869051967 (LCT-14009), rs145946881 (LCT-14010) amplified by Lac-1 primer set, and rs182549 (LCT-22018) by Lac-2 primer set. The sample S7 used in this experiment was obtained from the first extracts. PCR, polymerase chain reaction; NC, extraction negative control.
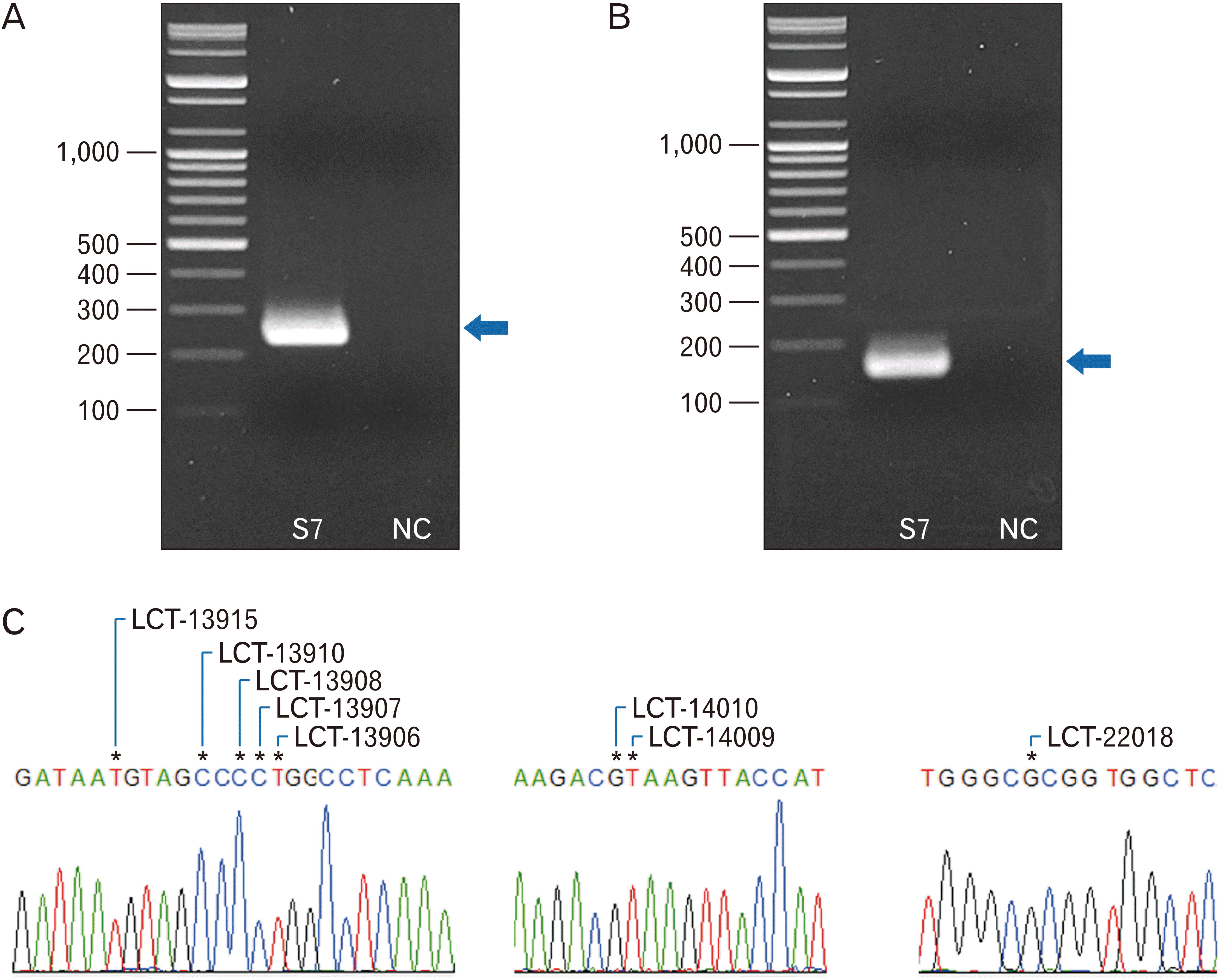
Table 1
The information of samples used in this study
Table 2
The information of primer sets and PCR condition
| Set | Primer | Sequence (5' to 3') | Product size (bp) | Annealing temperature (°C) | Reference |
|---|---|---|---|---|---|
| Lac1 | Lac1-F* | ACC CAC TGA CCT ATC CTC GT | 233 | 58 | This study |
| Lac1-R | ACG TCA TAG TTT ATA GAG TGC | ||||
| Lac2 | Lac2-F | TGG TCT CGA ACT CCT GAC | 147 | ||
| Lac2-R* | ACC CTA TCA GTA AAG GCC TA | ||||
| HV1 | F16112* | CAC CAT GAA TAT TGT ACG GT | 299 | 52 | [31] |
| R16410* | GAG GAT GGT GGT CAA GGG AC | ||||
| PCR condition (45 cycles) | Pre-denaturation at 95°C for 10 min | ||||
| Denaturation at 95°C for 20 sec | |||||
| Annealing for 30 sec | |||||
| Extension at 72°C for 20 sec | |||||
| Final extension at 72°C for 10 min | |||||
Table 3
Genotyping results on LNP SNPs in Joseon people
Table 4
Mitochondrial DNA haplotypes of Joseon individuals’ samples and researchers




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download