Abstract
Background/Aims
Methods
Results
Supplementary Material
Notes
Funding Source
This study was funded by Janssen Research & Development, LLC, Spring House, PA, USA and Janssen Pharmaceutical K.K., Tokyo, Japan.
Conflict of Interest
Hisamatsu T has received joint research support from Alfresa Pharma Co. Ltd; grant support from Mitsubishi Tanabe Pharma Corporation, EA pharma Co. Ltd., AbbVie GK, JIMRO Co. Ltd., Zeria Pharmaceutical Co. Ltd., Daiichi-Sankyo, Kyorin Pharmaceutical Co. Ltd., Nippon Kayaku Co. Ltd., Astellas Pharma Inc., Takeda Pharmaceutical Co. Ltd., Pfizer Inc., Mochida Pharmaceutical Co., Ltd.; consulting fee from EA pharma Co. Ltd., AbbVie GK, Celgene K.K., Janssen Pharmaceutical K.K., Pfizer Inc., Nichi-Iko Pharmaceutical Co., Ltd.; and lecture fee from Mitsubishi Tanabe Pharma Corporation, AbbVie GK, EA pharma Co. Ltd., Kyorin Pharmaceutical Co. Ltd., JIMRO Co., Janssen Pharmaceutical K.K., Mochida Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co. Ltd. Motoya S has received grant support from EA pharma Co. Ltd., AbbVie GK, Takeda Pharmaceutical Co. Ltd., Pfizer Inc. and lecture fee from Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co. Ltd., Suzuki Y has received honoraria from AbbVie, Mitsubishi Tanabe Pharma, ZERIA, Mochida Pharmaceutical Co. Ltd., Kyorin Pharmaceutical Co. Ltd., EA Pharma Co. Ltd., and commercial research funding from AbbVie, Mitsubishi Tanabe Pharma, EA Pharma Co. Ltd., JIMRO Co. Ltd., Mochida Pharmaceutical Co. Ltd., KISSEI, Nippon Kayaku. Ohnishi Y has received grant support from AbbVie GK. Fujii N, Matsushima N, and Zheng R are employees of Janssen Pharmaceutical K.K. Marano CW is an employee of Janssen R&D LLC, USA. Authors received the editorial support from SIRO Clinpharm Pvt. Ltd funded by Janssen Pharmaceutical K.K. Kim HJ has no conflict of interest to declare.
Author Contribution
Conceptualization: Hisamatsu T, Marano CW. Formal analysis: Fujii N, Matsushima N, Zheng R, Marano CW. Investigation: Hisamatsu T, Kim HJ, Motoya S, Suzuki Y, Ohnishi Y. Methodology: Fujii N, Matsushima N, Zheng R, Marano CW. Project administration: Fujii N. Visualization: Fujii N. Writing - original draft: Fujii N. Writing - review & editing: all authors. Approval of final manuscript: all authors.
Hisamatsu T was an investigator in the UNIFI Global Steering Committee. Kim HJ, Motoya S, Suzuki Y, and Ohnishi Y were investigators in the current study.
Others
The authors thank the study participants without whom this study would not have been accomplished, and also thank the investigators and study coordinators for their contributions to this study. Anupama Singh, SIRO Clinpharm Pvt. Ltd., provided medical writing assistance and Akino Tanaka, Janssen Pharmaceutical K.K., provided additional editorial support for this manuscript. Hiroki Yoshida (ex-employee of Janssen Pharmaceutical K.K.) provided statistical advice for this manuscript.
ACKNOWLEDGMENTS
REFERENCES
Fig. 1
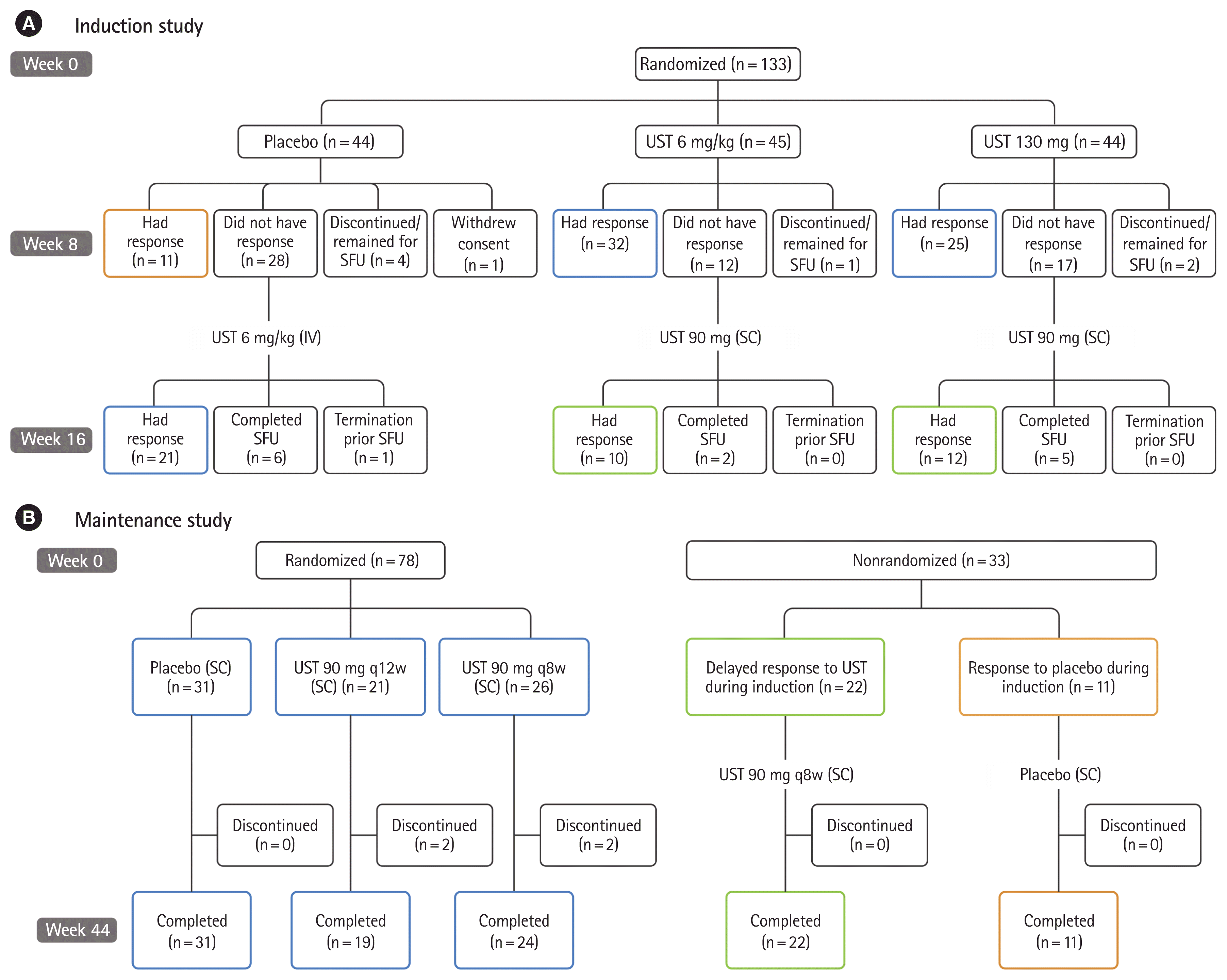
Fig. 2
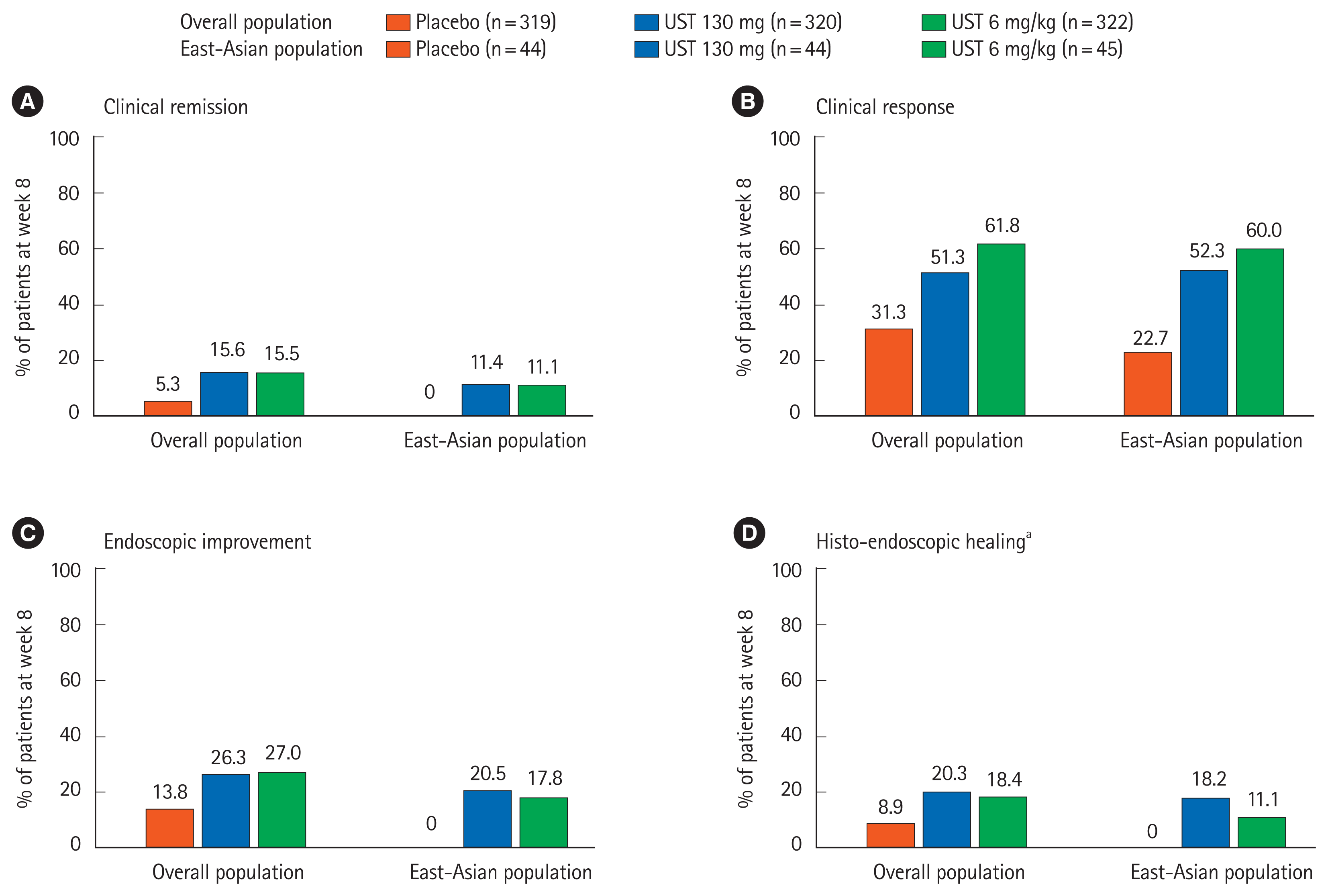
Fig. 3
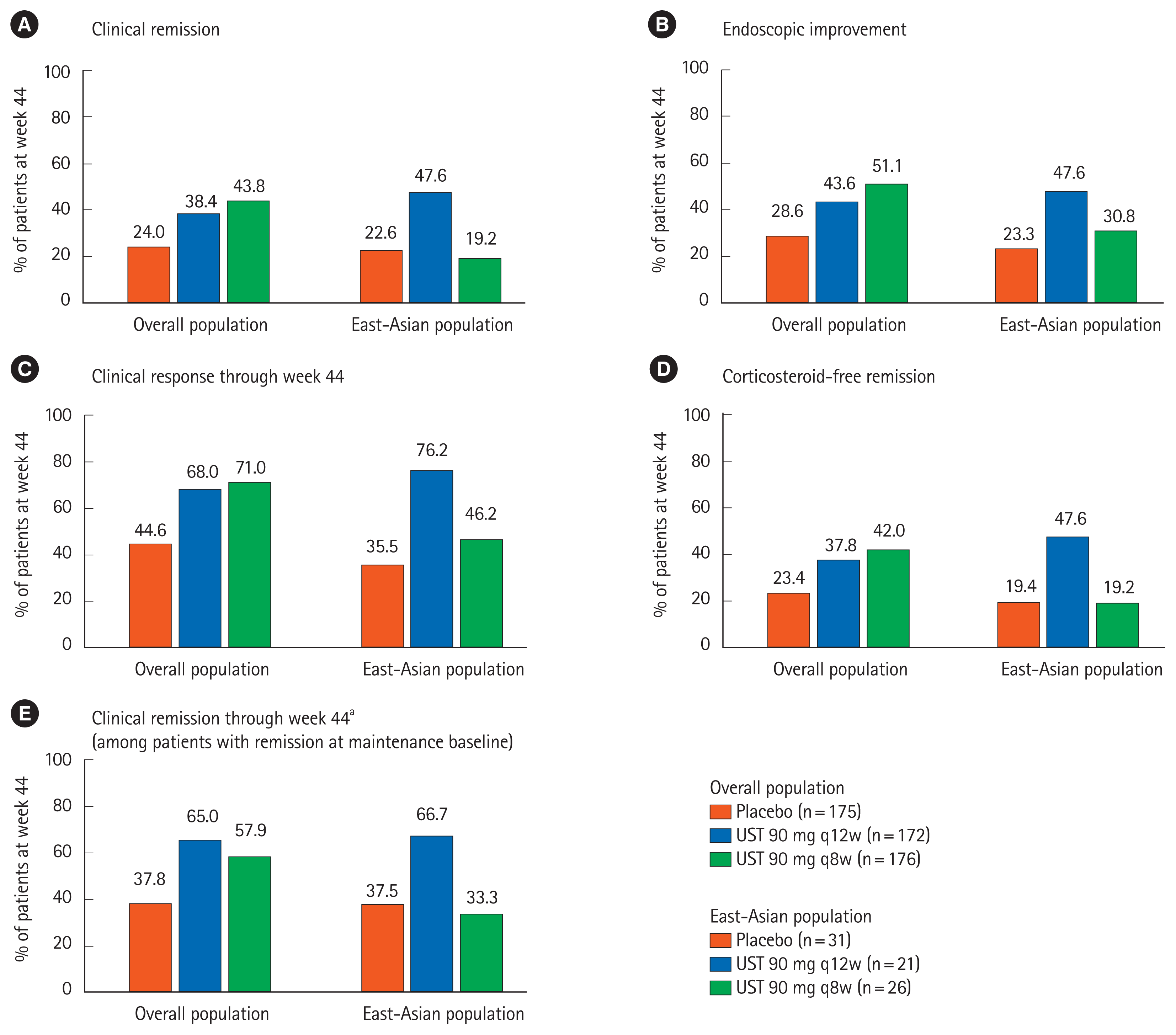
Table 1
| Characteristic | Induction study | Maintenance studya | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Placebo (n=44) | UST 130 mg (n=44) | UST 6 mg/kg (n=45) | Randomized | Nonrandomized | ||||
|
|
|
|||||||
| Placebo (n=31) | UST 90 mg (q12w) (n=21) | UST 90 mg (q8w) (n=26) | Responders to placebo (n=11) | Delayed responders (n=22) | ||||
| Male sex | 28 (63.6) | 32 (72.7) | 27 (60.0) | 23 (74.2) | 11 (52.4) | 11 (42.3) | 7 (63.6) | 19 (86.4) |
|
|
||||||||
| Age (yr) | 39.2±13.5 | 44.7±14.9 | 42.2±13.8 | 43.5±13.7 | 43.8±9.2 | 38.9±16.8 | 44.7±12.5 | 44.5±15.9 |
|
|
||||||||
| Body weight (kg) | 60.2±9.6 | 63.0±12.6 | 61.1±13.0 | 64.2±12.9 | 60.7±9.6 | 57.2±11.4 | 59.8±6.7 | 65.5±15.5 |
|
|
||||||||
| Disease duration (yr) | 7.3±4.9 | 8.1±6.7 | 8.1±6.5 | 7.1±6.0 | 8.9±6.5 | 8.8±6.6 | 8.0±4.9 | 9.3±6.8 |
|
|
||||||||
| Total Mayo score | 8.2±1.3 | 8.3±1.5 | 8.7±1.2 | 8.0±1.5 | 8.7±1.6 | 8.5±1.5 | 8.5±0.9 | 8.5±1.2 |
|
|
||||||||
| Disease severity | ||||||||
| Moderate (Mayo score 6–10) | 43 (97.7) | 41 (93.2) | 44 (97.8) | 30 (96.8) | 20 (95.2) | 25 (96.2) | 11 (100.0) | 21 (95.5) |
| Severe (Mayo score 11–12) | 1 (2.3) | 3 (6.8) | 1 (2.2) | 1 (3.2) | 1 (4.8) | 1 (3.8) | 0 | 1 (4.5) |
|
|
||||||||
| Extent of disease | ||||||||
| Extensive | 22 (50.0) | 19 (43.2) | 26 (57.8) | 17 (54.8) | 12 (57.1) | 14 (53.8) | 2 (18.2) | 11 (50.0) |
| Limited to left side of colon | 22 (50.0) | 25 (56.8) | 19 (42.2) | 14 (45.2) | 9 (42.9) | 12 (46.2) | 9 (81.8) | 11 (50.0) |
|
|
||||||||
| CRP (mg/L) | 1.4 (0.7–5.8) | 2.9 (0.9–10.7) | 3.6 (1.0–11.5) | 1.4 (0.3–7.2) | 1.7 (0.8–3.3) | 3.5 (1.3–9.8) | 4.3 (1.1–8.5) | 7.5 (1.2–13.3) |
|
|
||||||||
| Fecal calprotectin (mg/kg) | 737.5 (442.0–1,515.5) | 1,346.5 (231.0–1,964.0) | 1,217.0 (549.0–2,237.0) | 925.0 (323.0–1,532.0) | 1,202.0 (738.0–1,918.0) | 1,558.0 (663.0–3,199.0) | 746.0 (469.0–1,519.0) | 1,273.0 (542.0–2,237.0) |
|
|
||||||||
| Baseline UC medications | ||||||||
| Corticosteroid use | 15 (34.1) | 13 (29.5) | 19 (42.2) | 11 (35.5) | 6 (28.6) | 12 (46.2) | 5 (45.5) | 8 (36.4) |
| Immunomodulatory drugs | 25 (56.8) | 24 (54.5) | 16 (35.6) | 15 (48.4) | 10 (47.6) | 11 (42.3) | 4 (36.4) | 13 (59.1) |
|
|
||||||||
| History of biological treatment failure | 28 (63.6) | 28 (63.6) | 29 (64.4) | 20 (64.5) | 12 (57.1) | 19 (73.1) | 5 (45.5) | 18 (81.8) |
|
|
||||||||
| Patients with clinical remission at maintenance baselineb | - | - | - | 8 (25.8) | 3 (14.3) | 3 (11.5) | 0 | 1 (4.5) |
|
|
||||||||
| Patients with endoscopic healing at maintenance baselineb | - | - | - | 12 (38.7) | 4 (19.0) | 5 (19.2) | 0 | 3 (13.6) |
Table 2
| Variable | Induction study | Maintenance study | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Randomized | Nonrandomized | |||||||
|
|
|
|
||||||
| Placebo (n=44) | UST 130 mg (n=44) | UST 6 mg/kg (n=45) | Placebo (n=31) | UST 90 mg (q12w) (n=21) | UST 90 mg (q8w) (n=26) | Responders to placebo (n=11) | Delayed responders (n=22) | |
| Average duration of follow-up (wk) | 8.2 | 8.1 | 8.1 | 43.2 | 42.0 | 38.3 | 42.9 | 43.2 |
|
|
||||||||
| Any AE | 18 (40.9) | 19 (43.2) | 21 (46.7) | 28 (90.3) | 17 (81.0) | 19 (73.1) | 9 (81.8) | 17 (77.3) |
|
|
||||||||
| Serious AEs | 2 (4.5) | 1 (2.3) | 0 | 4 (12.9) | 1 (4.8) | 5 (19.2) | 1 (9.1) | 0 |
|
|
||||||||
| Infections | ||||||||
| Any | 8 (18.2) | 10 (22.7) | 9 (20.0) | 19 (61.3) | 7 (33.3) | 14 (53.8) | 6 (54.5) | 10 (45.5) |
| Serious | 1 (2.3) | 1 (2.3) | 0 | 1 (3.2) | 1 (4.8) | 1 (3.8) | 0 | 0 |
|
|
||||||||
| AEs leading to treatment discontinuation | - | - | - | 4 (12.9) | 0 | 2 (7.7) | 2 (18.2) | 1 (4.5) |
|
|
||||||||
| Malignancies/cancera | 0 | 0 | 0 | 0 | 0 | 1 (3.8) | 0 | 0 |
|
|
||||||||
| AEs within 1 hour of infusion | 0 | 1 (2.3) | 1 (2.2) | - | - | - | - | - |
|
|
||||||||
| AEs associated with injection-site reactions | - | - | - | 1 (3.2) | 0 | 0 | 0 | 0 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download