Abstract
Purpose
This prospective, single-center, open-label, therapeutic confirmatory, randomized clinical trial aimed to assess the alleviation of anal pain by applying structured anal skin care including skin protectants in rectal cancer patients with low anterior resection syndrome (LARS) combined with anal pain.
Methods
From December 2017 to May 2020, 42 patients with LARS (scores of ≥21) and anal pain (visual analogue scale [VAS] score of ≥3) were randomly assigned and observed for 4 weeks. The conventional treatment consisted of dietary management, sitz baths, prohibition of anal scrubbing, loperamide, and dioctahedral smectite. In the anal care group, cleanser, barrier cream, and barrier spray were applied to the anal skin after defecation following the conventional treatment. The primary outcome was analgesic effect on anal pain after 2 weeks of structured treatment (anal care group) or conventional (control group). The cutoff for analgesic effect was a decrease in the anal pain score (VAS score of ≥2 or ≥30% reduction).
Results
As a primary outcome, the analgesic effect was significantly higher in the anal care group (P = 0.034). The incontinence-associated dermatitis skin condition score was significantly improved in the anal care group than control group after 4 weeks (P = 0.023). There were no significant differences in LARS scores and quality of life scores between 2 groups.
Low anterior resection syndrome (LARS) is one of the most common uncomfortable symptoms experienced by patients undergoing low anterior resection (LAR) [1]. The symptoms of LARS may appear in various ways, such as urgency, frequency, tenesmus, loose stool, nocturnal defecation, and fecal incontinence [2]. The pattern and intensity of LARS symptoms may vary depending on various factors such as the length of the remnant rectum, radiation proctitis, diet, and bowel habits [3].
Loose stool and frequent defecation can commonly lead to anal dermatitis with pain, itching, pruritus, and a burning sensation. Loose stool contains water, electrolytes, bile, and digestive enzymes. Changes in alkaline pH can destroy the acidic mantle of the epidermis and initiate skin breakdown. Repetitive anal scrubbing and friction can cause physical damage to the anal skin. Loss of the skin barrier can cause bacterial and fungal infiltration into the skin, resulting in infection. These vicious cycles may lead to perianal dermatitis [45]. The mechanism of perianal dermatitis related to LARS is similar to that of incontinence-associated dermatitis (IAD) and peristomal dermatitis.
To alleviate the anal pain associated with LARS, dietary management, medication, and sitz baths are recommended after defecation. Using this conventional care, the symptoms of LARS could not be resolved in a short period and were gradually relieved over time [6]. Fecal incontinence-related symptoms could be improved with multimodal treatment including biofeedback therapy [78]. However, perianal dermatitis exacerbates severe anal pain and discomfort. Although perianal dermatitis and pain are very common after LARS, structured anal care has not been established for prompt pain relief.
Currently, stoma management is systematically performed by a wound, ostomy, and continence (WOCN) nurse in patients with rectal cancer [9]. The prevalence and severity of peristomal dermatitis and IAD were reduced, and dermatitis healing improved [10]. Since the mechanisms of peristomal dermatitis and perianal dermatitis are similar, it can be expected that skin protectant application around the perianal skin can be added to structured anal care programs to relieve anal pain quickly and effectively. However, there is still a lack of studies on standardized anal management for pain relief in perianal dermatitis following LARS.
This prospective, randomized clinical study aimed to assess the alleviation of anal pain by applying structured anal skin care including skin protectants to the anal skin in rectal cancer patients with LARS combined with anal pain.
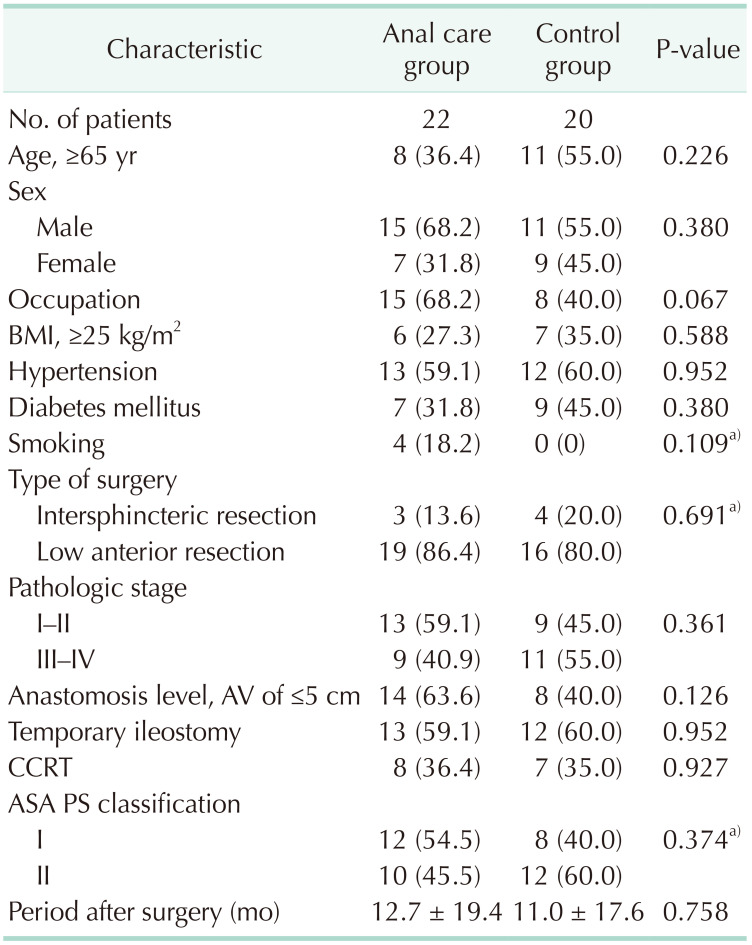
This study was conducted at Pusan National University Yangsan Hospital from January 2019 to December 31, 2020, after receiving the approval from Institutional Review Board (No. 05-2017-014). Informed consent was obtained from all the patients participating in the study. The inclusion criteria were patients who satisfied all the following criteria: (A) patient with rectal cancer who underwent LAR, (B) LARS score (≥21 points) and visual analog scale (VAS) score (≥3 points) for anal pain, (C) colorectal anastomosis location within 10 cm from the anal verge, and (D) ages of 18–85 years. The exclusion criteria were as follows: (A) abdominoperineal resection, (B) permanent stoma, (C) metastatic rectal cancer, (D) combined cancer other than colorectal cancer, (E) patients unable to complete questionnaires due to cognitive impairment, and (F) pregnant or lactating women.
In the anal care group, a structured skin care protocol was applied to relieve anal pain and enhance healing of perianal dermatitis that was based on recommended skin care protocol for IAD and peristomal dermatitis [11]. After defecation, a pH-balanced cleanser (Comfeel cleanser, Coloplast Co., Ltd., Humlebaek, Denmark) was used to remove urine and feces from the perianal skin. Skin moisturizing cream (Comfeel barrier cream, Coloplast Co., Ltd.) and skin protectant (Brava barrier spray, Coloplast Co., Ltd.) were applied around the anus after cleansing. Comfeel cleanser contains purified water, disodium cocoamphodiacetate, sodium lauryl sulfate, propylene glycol, allantoin, isopropyl alcohol, phenoxyethanol, methylparaben, ethylparaben, butylparaben, propylparaben, and fragrance. Comfeel cleanser can clean wounds without water and soap. Coconut oil (disodium cocoamphodiacetate) softens and protects the skin. Allantoin promotes the healing of damaged skin. The components of Comfeel barrier cream are pure water, mineral oil, petrolatum, ozokerite, glyceryl oleate, lanolin oil, propylene, glycol, glycerin, magnesium citrate, methylparaben, propylparaben, citric acid, and cyclomethicone. The barrier cream moisturizes and protects the skin by smoothing dry and sore skin, protecting it from maceration and irritation. The lanolin oil is an emollience. The glycerin ingredient penetrates deep into the skin to provide nutrition and moisturization, and the cyclomethicone ingredient takes a role as a protectant. The components of Brava barrier spray are disiloxane (90%) and decamethylcyclopentasiloxane (10%), and it is an oil and alcohol-free formulation. This siloxane mixture is a silicone-based product and dries quickly in seconds after application to protect the skin. Usual dosage for 1-time use is Comfeel cleanser of 5–10 mL (spray 2–3 times), Comfeel barrier cream of 2–3 g (cream capacity equivalent to squeezing toothpaste), and Brava barrier spray of 2–3 mL (apply 1–2 times).
Conventional management, including medication for loose stool, anal self-care, and dietary consultation, was administered to both control and anal care groups. Loperamide (Loperamide Cap, Samnam Pharm Co., Ltd., Geumsan, Korea) and dioctahedral smectite (Shumacton Susp Sachet 15% 20 mL, Ilyang Pharmaceutical Co., Ltd., Yongin, Korea) were prescribed to relieve the loose stools. The conventional self-care for anal skin management included sitz baths and prohibited scrubbing after defecation. A clinical nutritionist provided dietary advice to all patients to reduce the amount and frequency of stool and minimize colon irritation. A low-residual meal was recommended to reduce the amount of undigested residues, such as coarse fibers. The patients were asked to chew food slowly enough and maintain regular meal times. In addition, excessively sweet, spicy, or oily foods could cause loose stool or diarrhea; therefore, the intake of these foods was limited. The patients were asked to drink 1.5–2 L of water per day.
The patients were randomly assigned using a computerized random number table. Random sequence generation for allocation was performed by an independent statistician using the Excel program ver. 16.0 (Microsoft, Redmond, WA, USA). The allocation table was sealed in an opaque envelope for allocation concealment. The patients were randomized using an individual randomization number by an independent investigator who was not involved in the outcome assessments.
An assessment investigator was blinded to the allocation and evaluated the VAS score for anal pain, LARS score, IAD score, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ) C-30 as quality of life (QoL) score, and patient satisfaction. Surveys were conducted at visit 1 (V1, study participation date), visit 2 (after 1 week), visit 3 (after 2 weeks), and visit 4 (after 4 weeks). Anal pain relief and patient satisfaction were investigated at visits 2, 3, and 4 (Supplementary Table 1).
The intensity of anal pain was recorded using a 10-point VAS scale. Anal pain was defined as a VAS score of ≥3. The LARS questionnaire was used for LARS score measurement (range, 0–4). LARS was defined as a LARS score of ≥21 [112].
The IAD score for anal skin condition was evaluated by a WOCN nurse using the IAD skin condition assessment tool of Kennedy and Lutz [13]. The IAD score (range, 0–9) was counted by combining the scales of damaged skin (range, 0–3), skin erythema (range, 0–3), and erosion (range, 0–3). The EORTC QLQ C-30 questionnaire was used to assess patients’ QoL. The higher the functional and overall health scores, the higher the QoL, and the higher the symptom experience score (range, 0–100), the lower the QoL [14].
A compliance questionnaire was used to evaluate treatment compliance. Patient satisfaction with treatment was investigated using a satisfaction questionnaire.
The primary outcome was whether anal pain was relieved after 2 weeks of treatment in patients with anal pain after LAR. An acceptable analgesic effect was defined when the anal pain score decreased by 2 points or 30% or more. The secondary outcomes included anal pain score, IAD score, LARS score, QoL score, treatment compliance, and satisfaction with treatment.
However, little is known about the response rate of anal pain after LAR using conventional anal management and drug therapy. As a result of our preliminary study, most anal pain relief was achieved at the 10th–12th week after LAR, and pain relief was achieved in approximately 30.0% of patients at 2nd week after surgery. Therefore, it is assumed that the response rate for relieving anal pain in the control group was 30.0% in the conventional anal care group, and the response rate in the anal care group reached 70.0% after the skin protection agent was additionally applied to the anus. When the significance level was 5.0%, the power was 80.0%, and the dropout rate was 10.0%, 23 patients were calculated as requirements in each group for a total of 46 patients.
Results are expressed as mean ± standard deviation or number (%). Continuous variables were analyzed using repeated measures analysis of variance (RM-ANOVA). Categorical variables were analyzed using the Pearson chi-square and Fisher exact tests, and Spearman correlation analysis was used to find the correlation between anal pain score and LARS score. RM-ANOVA was used to assay patient groups (anal care and control), treatment time (baseline, 1st week, 2nd week, and 4th week), and interaction as anal pain score, IAD score, LARS score, and QoL score. IBM SPSS Statistics ver. 26 (IBM Corp., Armonk, NY, USA) and R software ver. 4.1.1 (RStudio, Boston, MA, USA) was used for the statistical analysis.
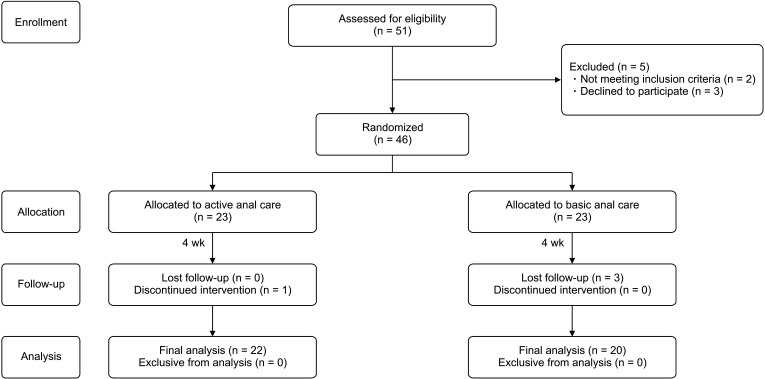
The 46 patients were enrolled in this study and 42 patients were included in the analysis (Fig. 1). The reasons for dropping out were voluntary withdrawal (1 patient) and difficulties in following the outpatient visit schedule as weekly meetings (3 patients).
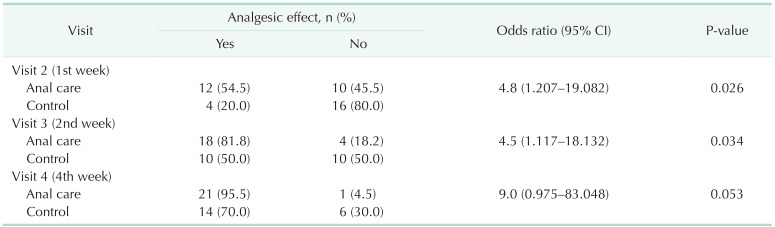
The clinical characteristics of the anal care and control groups did not differ significantly (Table 1). There were no adverse reactions caused by the structured skincare treatments during the study. As a primary outcome, the analgesic effect in the anal care group at visit 3 (2nd week) was significantly higher than that in the control group (P = 0.034) (Table 2).
The anal pain score showed a significant decrease until 4 weeks of treatment in both the anal care and control groups. At visit 2 (1st week), anal pain was significantly lower in the anal care group (P = 0.006). In both groups, the condition of the anal skin improved over time. The IAD scores decreased steeply in the experimental group. At visit 3 (2nd week) and visit 4 (4th week), the IAD scores of the anal care group were significantly improved compared to that of the control group (Fig. 2).
RM-ANOVA shows that the interaction between group and time was statistically significant for anal pain score (P = 0.002) and IAD score (P = 0.010). On the other hand, the LARS score was not significant for group (Table 3). Significant correlations were observed between the anal pain score and LARS score (r = 0.41, P = 0.008) in visit 1 (baseline). But these correlations became insignificant after structured or conventional anal care, as the anal pain was relieved and LARS still persisted for 4 weeks (Fig. 3).
The QoL questionnaire was divided into general health status, functional status, and symptom experience level. In the overall item reliability analysis, Cronbach alpha value was 0.906, confirming internal consistency. The reliability alpha value of the global health status was 0.879. For the functional and symptom scales, the reliability alpha values were 0.908 and 0.823, respectively. Global health status and functional status including physical, role, emotional, and cognitive functioning parameters were significantly improved during 4 weeks of treatment in both groups. Symptom experiences including fatigue, pain, insomnia, appetite loss, diarrhea, and financial difficulties were also significantly improved in both groups. On the RM-ANOVA analysis, there were no statistical differences in global health status, functional status, and symptom experience except appetite loss (visit 2) between the anal care and control groups (Fig. 4).
Compliance with treatment was similar between the 2 groups. The compliance rates for anal skin care ranged from 72.7% to 75.4% (Supplementary Table 2). In the treatment satisfaction survey, the anal care group showed significantly higher satisfaction than the control group. In the anal care group, the convenience of structured skin care treatment was maintained at approximately 80% during the treatment period (Supplementary Table 3).
For anal pain and perianal dermatitis after LAR, analgesic effect of structured skin care program was compared with conventional anal management in this study. Anal pain significantly reduced from the 1st week in patients who underwent structured skin care. The analgesic effect at 2 weeks, which was the primary outcome of this study, was significantly higher in the anal care group. The IAD skin condition assessment score, indicating the severity of perianal dermatitis, showed a significant improvement after 4 weeks of treatment in the anal care group. To the best of our knowledge, this is the first randomized clinical trial to evaluate the analgesic efficacy of structured anal skin care on anal pain and perianal dermatitis in rectal cancer patients with LARS.
LARS following rectal cancer surgery causes a change in bowel function in almost all patients [15]. Unfortunately, the cause of LARS, which disrupts a patient’s daily life, is still not fully understood [1617]. Various causes have been considered, such as imbalance of bowel movement due to changes in the autonomic nervous system that controls bowel movements, excessive colonic contraction, and loss of defecation reservoir volume. The symptoms of LARS also appear in various ways, such as frequent stools, tenesmus, and fecal incontinence [18]. Although drug treatment for these symptoms has been attempted, LARS still torments many patients with rectal cancer.
Frequent and loose stools are common symptoms and are often accompanied by anal pain and perianal dermatitis after surgery. The incidence and pathophysiologic mechanism of anal pain and perianal dermatitis can only be estimated based on IAD and peristomal dermatitis, which have an approximately similar clinical course [19]. Therefore, treatment for perianal dermatitis has been applied similarly to IAD. However, scientific research and interest from the surgical community are still lacking on anal pain and perianal dermatitis in patients with LARS; therefore, structured and standardized anal skin care has not been established.
Anal pain accompanying LAR can have a variety of causes. If the colorectal anastomotic stapling line is close to the anal canal, the sphincter muscle may partially be included in the stapling line, causing intractable anal discomfort. Severe postoperative pain was reported in some patients who underwent stapled hemorrhoidectomy [20]. However, no anal sphincter was included in the stapling line in this study. Various anal diseases, such as 4th-degree hemorrhoids, anal fissures, and anal fistulas, can also cause anal pain. In this study, when a patient complained of anal pain, it was checked whether anal disease was present, and none of the patients complained of anal disease. In this study, anal pain in patients with LARS was limited to cases associated with perianal dermatitis caused by frequent loose stools after LAR.
Perianal dermatitis in LARS is a common discomfort in clinical settings; however, its pathophysiological mechanism is not yet known. Fortunately, perianal dermatitis in LARS could occur with a mechanism similar to that of IAD, which is related to urinary and fecal incontinence [21].
Urine contains urea and ammonia, which induce an alkaline pH in the skin from weak acids, thereby lowering the skin’s defense mechanism. When the anal skin is exposed to loose stool, the acidic mantle of the skin’s stratum corneum becomes weaker because of the alkaline component and the digestive enzymes contained in the stool, causing dermatitis. The urease secreted by fecal bacteria converts urea into ammonia, and alkaline conditions can induce the activation of lipolytic and proteolytic enzymes that destroy the lipid layer and corneocytes of the stratum corneum, thus continuing the vicious cycle of perianal dermatitis. In addition, a large amount of digestive enzymes remaining in the loose stool directly damages skin epithelial cells. Furthermore, repeated scrubbing on weakened skin can cause physical skin damage that drives the progression of dermatitis [42223].
The loose stool with alkaline pH can destroy the acidic skin circumstances. However, the pH of ileostomy component is 6.3, slightly acidic. In the range of normal feces, it is known that pH of 7.0–7.5, slightly acidic. In the incontinence patients, alkaline pH status was established in the skin by alkaline component of loose stool and ammonia converted by bacterial protease and urease [4]. In this study, it was not possible to measure the pH of the stool or skin of the patients. However, as the first process that causes skin damage in IAD patients, initial damage process is considered as disruption of the skin's acidic mantle. Therefore, the cleanser is a weakly acidic solution that stabilizes a pH balance according to the alkaline condition of the skin and relieves the skin discomfort caused by chemical irritation with alkaline compound, thereby reducing anal pain.
During outpatient follow-up after LAR, it is common to experience sudden pain in the anus, even if frequent and loose stools occur temporarily after an oily or spicy diet. The sudden occurrence of anal pain due to loose and frequent stools can be explained by the mechanism by which IAD occurs within a short period of a few days. In previous studies, IAD was initiated by fecal incontinence within a few hours. Therefore, perianal dermatitis of LARS, which has a mechanism similar to that of IAD, also occurs easily with loose stools and repeated friction, which can often cause anal pain abruptly in rectal cancer patients [24]. Studies on IAD have mainly been conducted on long-term care inpatients in nursing hospitals, severely paralyzed patients with reduced mobility, and immobile patients in intensive care units [25]. Interestingly, the time required for urinary and fecal incontinence to cause dermatitis was very short (1–3 days). IAD occurred in 54% of inpatients who did not receive active perineal care within 72 hours. However, the frequency of IAD was reduced to 3% in the patient group who actively performed perineal care [26].
The patients in this study were different from those who were seriously ill, immobilized, or had cognitive impairment. In this study, the patients were able to follow up in an outpatient clinic after surgery and did not have cognitive impairments. Therefore, they were able to maintain self-management for anal care, such as sitz baths and avoidance of anal scrubbing. Moreover, all patients who participated in this study received diet counseling from a clinical dietitian. All patients with loose and frequent stools were treated with drug treatment using loperamide and dioctahedral smectite. In particular, the patients were classified as having mild perianal dermatitis without bacterial or fungal skin infections.
Anal pain could be relieved to some extent by using sitz baths and oral analgesics. In the past, the symptomatic treatment for acute anal pain was anal self-management with an additional prescription of loperamide and NSAIDs. However, anal pain continues until perianal dermatitis recovers. Therefore, it is necessary to add structured skin protection to relieve anal pain associated with perianal dermatitis quickly. However, there are few studies on topical formulations that can be directly applied to dermatitis to obtain an immediate analgesic effect in rectal cancer patients with LARS. Therefore, our findings are very encouraging in that prompt anal pain relief effect was significantly improved in the anal care group that additionally received structured anal skin care compared with patients who received conventional care in patients with LARS and mild perianal dermatitis.
Fortunately, many studies have been conducted on structured skin care for the treatment of peristomal dermatitis and IAD [27]. Skin protectant treatment consists of 3 steps: skin cleansing to remove dirty contaminants, lipid (emollient) and moisture (humectant) supply, and skin protection. Soaps and water are often used to remove skin contaminants. Most soaps are made of weak alkali, which can exacerbate the weak alkali state and further stimulate dermatitis. Therefore, it is necessary to use a solution with a slightly acidic to neutral pH to remove skin contamination. Emollients can supply skin lipids that help restore the protective function of the skin. In healthy skin, free fatty acid and cholesterol from the sebaceous glands spread out on the skin surface to prevent excessive evaporation of moisture and protect skin cells. The moisture of the skin cells must be properly maintained; however, when the skin is exposed to excessive moisture, the skin protection mechanism is damaged due to the swelling of the stratum corneum. Conversely, when the moisture content in the skin decreases in the elderly, dry skin cannot maintain the skin protection mechanism and dermatitis easily occurs. A skin moisturizer has 2 functions: supply adequate moisture to the skin’s surface layer and block excessive moisture that penetrates from the outside [27]. Advanced skin protectants are made of silicone or polymer, which can be used to form a physical protective layer and can be applied in the form of a spray or film [28].
In this study, the analgesic effects of a skin protectant for perianal dermatitis-related anal pain were evaluated by applying a skin protectant to peristomal dermatitis and IAD. The analgesic effect, which was the primary outcome of this study, was significantly improved in the anal care group to which the skin protectant was applied, compared with the control group. In particular, the pain relief effect was evident from the 1st week of treatment. The pain relief effect appeared in both the control and anal care groups after 4 weeks of treatment, but there was no statistically significant difference between the 2 groups. Interestingly, the IAD skin condition assessment score, which reflects the degree of dermatitis, did not differ between the 2 groups at the 1st week of treatment but was significantly improved in the anal care group at the 4th week of treatment. In other words, the pain relief effect of the skin protectant appeared rapidly from the 1st week, but the improvement in dermatitis showed a significant difference at the 4th week of treatment. Therefore, the rapid pain relief effect of skin protectants can quickly relieve patient discomfort. Perianal dermatitis recovered slowly over the course of treatment and improved significantly in the anal care group at the 4th week of treatment.
In this study, the period of assessment was determined within 4 weeks after the treatment. The various symptoms of LARS continue after surgeries, but anal pain and its response to structured anal care was the primary outcome. Pain relief could be achieved quickly when the patient responds to topical skin protectants. Wound healing process also has inflammatory stage (0–3 days), proliferative stage (1–2 weeks) following maturation stage after 2 weeks. So, the response determination of anal pain relief and inflammatory wound healing process of perianal dermatitis could be possible within 4 weeks. Perianal skin injury caused by frequent loose stool in LARS causes anal pain after defecation, and the topical skin protectants were expected to have an immediate pain relief effect. Our previous preliminary study showed pain relief in 1–2 weeks. Pain relief was achieved quickly when a skin protectant was used. Therefore, it is considered that 1–2 weeks is sufficient for the study period to compare the analgesic effect of anal pain. So, the primary outcome of this study was the analgesic effect at the 2nd week. The dermatitis requires a pathologically healing period, and it was expected that the recovery period would take more than 1–2 weeks. Therefore, the dermatitis score was evaluated for up to 4 weeks in this study, and the IAD scores at 3rd and 4th weeks showed a statistically significant improvement in the anal care group. It was found that the analgesic effect appeared immediately, while the dermatitis healing process took place slowly for 4 weeks.
It is important to determine the dose and frequency of use for skin care products such as pH-cleanser, skin moisturizing cream, and skin protectant for clinical trials. When the patients in the anal care group had defecation, they were asked to wash the anus with water and pH-balanced cleanser. After washing anus, skin barrier cream was applied. The skin barrier spray was applied at least once a day. The skin barrier spray could be applied by a family when it was difficult for the patient to use it alone. Usual dosage for 1-time use is pH-balanced cleanser of 5–10 mL, barrier cream of 2–3 g, and barrier spray of 2–3 mL. As a survey of treatment compliance, about 72% of the patients continued to use the skin protectant. The statistical analysis was performed by the intention-to-treat analysis and included the patients who did not use skin protectants among the patients assigned to the anal care group. In the control group, conventional anal care was performed in the same manner except for the skin protectant. We tried to keep the protocol of the anal care group and the control group the same as possible. However, patients’ compliance with anal skin care appeared to be as follows: medication, 87%–97%; dietary management, 75%–84%; and self-anal care, 72%–83%. There was no statistical difference in compliance of the anal care treatment protocol between the anal care group and the control group during the outpatient follow-up period (Supplementary Table 2). At the 4th week, the administration of loperamide and dioctahedral smectite was significantly higher in the anal care group than in the control group (95.2% vs. 87.0%, respectively; P = 0.008). Because pain relief at the 2nd week was the primary outcome in this study, the difference in drug intake at the 4th week did not affect the primary outcomes. In addition, there was no chance to use the skin protectant in the control group during study period.
As expected, the anal pain relief effect of skin protectants was not related to the improvement in the LARS score. There was no significant difference in LARS scores between the anal care and control groups. Medications including loperamide, dioctahedral smectite, atropine sulfate, serotonin antagonists, and probiotics could be administered for LARS treatment, but it takes a long time to improve various symptoms, such as frequent bowel movements, and loose stools. However, this study indicates that anal pain can be quickly relieved by the application of topical skin protectants, even when various symptoms of LARS continue.
The QoL scores improved significantly in both the anal care and control groups during the 4 weeks of treatment. However, there was no significant difference in the QoL scores between the anal care and control groups. The analgesic effect of anal pain relief is still limited by further improvement in the overall QoL indicators. Therefore, long-term treatment using a multidisciplinary approach is required for patients after LARS [21]. In addition, if various symptoms of LARS are alleviated through a comprehensive and well-organized patient-specific treatment strategy, QoL will ultimately be improved [29].
This study had some limitations. Since this was a small-scale single-center study conducted at a single institution, the results of this study need to be verified by a large-scale multicenter clinical trial. This open-label study could not avoid performance bias, as it was impossible to blind the patients for allocation. Therefore, investigators assessing anal pain and perianal dermatitis were blinded to the randomized assignments to minimize detection bias. In future studies, a double-blinded design using placebo is needed to verify the efficacy of skin cleansers, barrier creams, and skin protectants in patients with LARS and perianal dermatitis. The skin condition assessment tool for dermatitis is still under development and is expected to be verified for the establishment of a standardized assessment tool to evaluate perianal dermatitis [30].
In conclusion, structured anal skin care has a significant analgesic effect in reducing anal pain and improving anal skin condition in patients with LARS after rectal cancer surgery. Therefore, systematic and standardized management of perianal skin is expected to improve perianal dermatitis in patients with rectal cancer with LARS.
ACKNOWLEDGEMENTS
The researchers appreciate Coloplast Korea for its cooperation in providing medical supplies for skin care.
Notes
References
1. Kim CW, Jeong WK, Son GM, Kim IY, Park JW, Jeong SY, et al. Validation of Korean version of low anterior resection syndrome score questionnaire. Ann Coloproctol. 2020; 36:83–87. PMID: 32054239.
2. Cura Pales CG, An S, Cruz JP, Kim K, Kim Y. Postoperative bowel function after anal sphincter-preserving rectal cancer surgery: risks factors, diagnostic modalities, and management. Ann Coloproctol. 2019; 35:160–166. PMID: 31487762.
3. Liang LS, Zain WZ, Zahari Z, Zakaria AD, Hashim MN, Wong MP, et al. Risk factors associated with low anterior resection syndrome: a cross-sectional study. Ann Coloproctol. 2022; 06. 03. DOI: 10.3393/ac.2022.00227.0032. [Epub].
4. Beeckman D, Schoonhoven L, Verhaeghe S, Heyneman A, Defloor T. Prevention and treatment of incontinence-associated dermatitis: literature review. J Adv Nurs. 2009; 65:1141–1154. PMID: 19374674.
5. Gray M, Bliss DZ, McNichol L. Moisture-associated skin damage: expanding and updating practice based on the newest ICD-10-CM codes. J Wound Ostomy Continence Nurs. 2022; 49:143–151. PMID: 35255065.
6. Yoo RN, Kye BH, Kim H, Kim G, Cho HM. The pattern of bowel dysfunction in patients with rectal cancer following the multimodal treatment: anorectal manometric measurements at before and after chemoradiation therapy, and postoperative 1 year. Ann Coloproctol. 2022; 03. 11. DOI: 10.3393/ac.2021.00696.0099. [Epub].
7. Lee KH, Kim JS, Kim JY. Efficacy of biofeedback therapy for object ive improvement of pelvic function in low anterior resection syndrome. Ann Surg Treat Res. 2019; 97:194–201. PMID: 31620393.
8. Weledji EP. Electrophysiological basis of fecal incontinence and its implications for treatment. Ann Coloproctol. 2017; 33:161–168. PMID: 29159162.
9. McNichol L, Bliss DZ, Gray M. Moisture-associated skin damage: expanding practice based on the newest ICD-10-CM codes for irritant contact dermatitis associated with digestive secretions and fecal or urinary effluent from an abdominal stoma or enterocutaneous fistula. J Wound Ostomy Continence Nurs. 2022; 49:235–239. PMID: 35523238.
10. Budri AM, McEvoy NL. Moisture-associated skin damage: a framework to guide decision making. Br J Nurs. 2022; 31:S4–S6.
11. Voegeli D, Hillery S. Prevention and management of moisture-associated skin damage. Br J Nurs. 2021; 30:S40–S46.
12. Buzatti KC, Petroianu A, Laurberg S, Silva RG, Rodrigues BD, Christensen P, et al. Validation of low anterior resection syndrome score in Brazil with Portuguese. Ann Coloproctol. 2022; 05. 13. DOI: 10.3393/ac.2022.00136.0019. [Epub].
13. Langemo D, Hanson D, Hunter S, Thompson P, Oh IE. Incontinence and incontinence-associated dermatitis. Adv Skin Wound Care. 2011; 24:126–140. PMID: 21326024.
14. Kaasa S, Bjordal K, Aaronson N, Moum T, Wist E, Hagen S, et al. The EORTC core quality of life questionnaire (QLQ-C30): validity and reliability when analysed with patients treated with palliative radiotherapy. Eur J Cancer. 1995; 31A:2260–2263. PMID: 8652253.
15. Pieniowski EH, Nordenvall C, Palmer G, Johar A, Tumlin Ekelund S, Lagergren P, et al. Prevalence of low anterior resection syndrome and impact on quality of life after rectal cancer surgery: population-based study. BJS Open. 2020; 4:935–942. PMID: 32530135.
16. van der Heijden JA, van de Pas KG, van den Broek FJ, van Dielen FM, Slooter GD, Maaskant-Braat AJ. Oncological and functional outcomes of transanal total mesorectal excision in a teaching hospital in the Netherlands. Ann Coloproctol. 2021; 38:28–35. PMID: 34182715.
17. Ha RK, Park SC, Park B, Park SS, Sohn DK, Chang HJ, et al. Comparison of patient-reported quality of life and functional outcomes following laparoscopic and transanal total mesorectal excision of rectal cancer. Ann Surg Treat Res. 2021; 101:1–12. PMID: 34235111.
18. Dulskas A, Kavaliauskas P, Pilipavicius L, Jodinskas M, Mikalonis M, Samalavicius NE. Long-term bowel dysfunction following low anterior resection. Sci Rep. 2020; 10:11882. PMID: 32681140.
19. Parnham A, Copson D, Loban T. Moisture-associated skin damage: causes and an overview of assessment, classification and management. Br J Nurs. 2020; 29:S30–S37.
20. Feng J, Cheng J, Xiang F. Management of intractable pain in patients treated with hemorrhoidectomy for mixed hemorrhoids. Ann Palliat Med. 2021; 10:479–483. PMID: 33545778.
21. Christensen P, Im Baeten C, Espín-Basany E, Martellucci J, Nugent KP, Zerbib F, et al. Management guidelines for low anterior resection syndrome: the MANUEL project. Colorectal Dis. 2021; 23:461–475. PMID: 33411977.
22. Beeckman D, Woodward S, Gray M. Incontinence-associated dermatitis: step-by-step prevention and treatment. Br J Community Nurs. 2011; 16:382–389. PMID: 21841630.
23. Beeckman D. A decade of research on incontinence-associated dermatitis (IAD): evidence, knowledge gaps and next steps. J Tissue Viability. 2017; 26:47–56. PMID: 26949126.
24. Park KH, Kim KS. Effect of a structured skin care regimen on patients with fecal incontinence: a comparison cohort study. J Wound Ostomy Continence Nurs. 2014; 41:161–167. PMID: 24343030.
25. Warshaw E, Nix D, Kula J, Markon CE. Clinical and cost effectiveness of a cleanser protectant lotion for treatment of perineal skin breakdown in low-risk patients with incontinence. Ostomy Wound Manage. 2002; 48:44–51.
26. Pather P, Hines S, Kynoch K, Coyer F. The effectiveness of topical skin products in the treatment and prevention of incontinence-associated dermatitis: a systematic review protocol. JBI Database System Rev Implement Rep. 2015; 13:36–52.
27. Chen Y, Gao Y, Zhang J, Niu M, Liu X, Zhang Y, et al. Quality and clinical applicability of recommendations for incontinence-associated dermatitis: a systematic review of guidelines and consensus statements. J Clin Nurs. 2022; 04. 11. DOI: 10.1111/jocn.16306. [Epub].
28. Lager P, Loxdale L. Use of breathable silicone technology in an ostomy appliance flange. Br J Nurs. 2021; 30(Suppl 8):25–35. PMID: 34106774.
29. Ridolfi TJ, Berger N, Ludwig KA. Low anterior resection syndrome: current management and future directions. Clin Colon Rectal Surg. 2016; 29:239–245. PMID: 27582649.
30. Beeckman D, Van den Bussche K, Alves P, Arnold Long MC, Beele H, Ciprandi G, et al. Towards an international language for incontinence-associated dermatitis (IAD): design and evaluation of psychometric properties of the Ghent Global IAD Categorization Tool (GLOBIAD) in 30 countries. Br J Dermatol. 2018; 178:1331–1340. PMID: 29315488.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1–3 can be found via https://doi.org/10.4174/astr.2022.103.6.360.
Supplementary Table 2
The questionnaires for patient’s compliance with treatments (n = 42)
Supplementary Table 3
The questionnaires for patient’s satisfaction with treatment (n = 42)
Fig. 2
The anal pain and skin condition changes for 4 weeks of anal care treatment. (A) The anal pain scores using the pain visual analog scale in the anal care group (solid line) and control group (dotted line). (B) Skin dermatitis score using incontinence-associated dermatitis (IAD) skin condition assessment tool designed by Kenney and Lutz [13]. Repeated measures analysis of variance shows that the interaction between group and time was statistically significant for anal pain score (P = 0.002) and IAD score (P = 0.010). Time, P-value over treatment time course; Group, P-value comparing between anal care and control groups; Time × group, P-value of interactions between treatment time and patient groups. *P < 0.05.
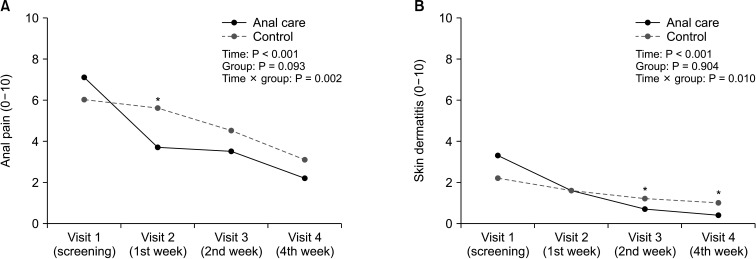
Fig. 3
The correlation between patient’s anal pain score and low anterior resection syndrome (LARS) score at each visit points. Significant correlations were observed between the anal pain score and LARS score (r = 0.41, P = 0.008) in visit 1 (baseline). But these correlations became insignificant after structured or conventional anal care.

Fig. 4
The quality of life (QoL) assessment in the anal care and control groups using EORTC QLQ C-30 score. The mean value of each QoL score was indicated in the anal care group (solid line) and control group (dotted line). Repeated measures analysis of variance was used for analysis of the interaction between treatment time and patient groups. EORTC QLQ, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire. Time, P-value over treatment time course; Group, P-value comparing between anal care and control groups; Time × group, P-value of interactions between treatment time and patient groups. *P < 0.05
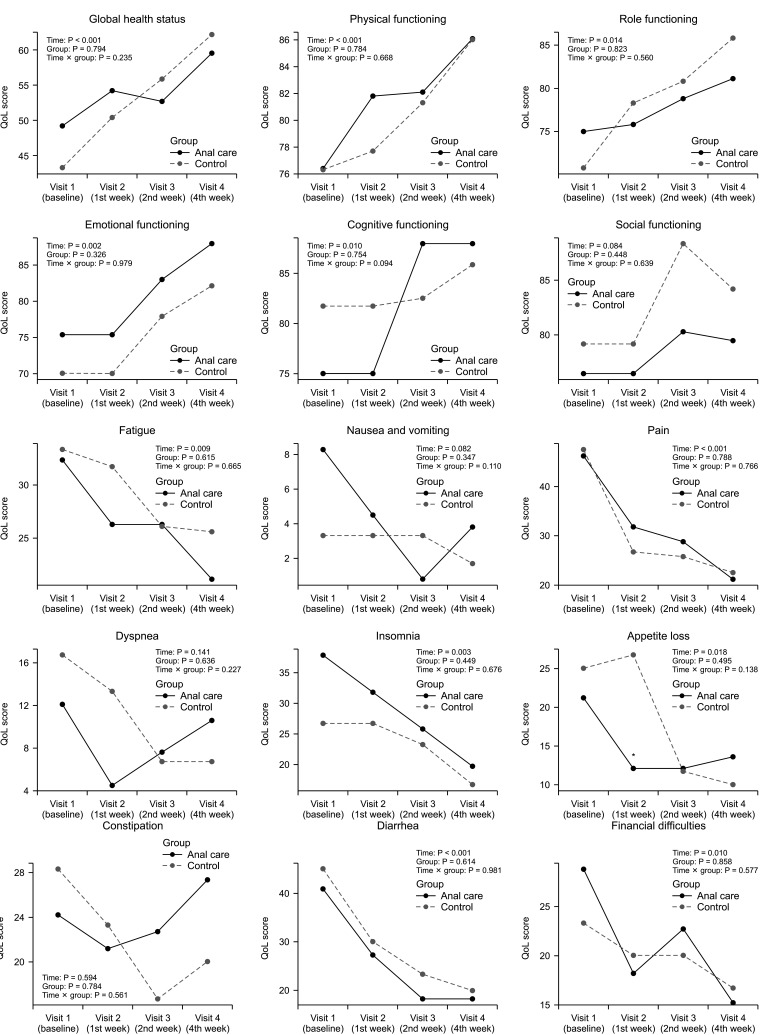
Table 3
Comparison of anal pain, pain relief, perianal dermatitis, and LARS score in the anal care and control groups
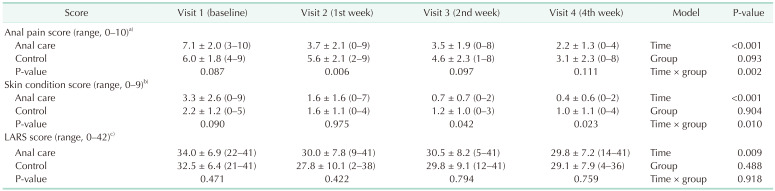
Values are presented as mean ± standard deviation (range).
LARS, low anterior resection syndrome.
Repeated measures analysis of variance was used for analysis model for treatment time, patient groups (anal care and control), and interaction for anal pain score, incontinence-associated dermatitis (IAD) score, and LARS score.
a)Visual analog scale score; b)cumulative score calculated with IAD skin condition assessment tool; c)LARS questionnaire score.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download