Abstract
As most individuals acquire immunity to severe acute respiratory syndrome coronavirus 2, South Korea declared a return to normalcy a few months ago. However, epidemic waves continue because of endlessly emerging variants and waning immunity. Health authorities are focusing on those at high risk of severe coronavirus disease 2019 to minimize damage to public health and the economy. In this regard, we investigated the vaccination rates in patients with various chronic medical conditions by examining the national health insurance claims data and the national immunization registry. We found that patients with chronic medical conditions, especially those of higher severity, such as malignancy, had vaccination rates approximately 10–20% lower than those of the general population. Public health authorities and healthcare providers should try to vaccinate these patients to avoid preventable morbidity and mortality.
Graphical Abstract
Shortly after the report of the first case of coronavirus disease 2019 (COVID-19) in South Korea,1 various preventive measures were implemented to protect those at a high risk of developing severe COVID-19, especially those admitted to hospitals or long-term care facilities. A high level of social distancing was enforced for 2 years; however, quarantine strategies changed after the end of the large pandemic wave of the omicron variant in early 2022. By mid-April 2022, virtually all restrictions were lifted in the context of adequate population immunity, achieved by a high vaccination rate and previous infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Owing to public fatigue and decoupling between case incidence and mortality, nationwide lockdown or intensive social distancing is unlikely to return. However, in the face of the incessant emergence of highly transmissible immune-escaping variants and waning population immunity, the outbreak of another epidemic is only a matter of time and is likely to cause significant morbidity and mortality in those at a high risk of severe infection. Sublineages of the omicron variant, BA.4 and BA.5, have already caused an alarming number of infection.2 Hence, we should be prepared to minimize the damage caused by future pandemic waves.
People who visit healthcare institutions because of chronic medical conditions are not only vulnerable to severe infection but are also likely to transmit the virus to other frail patients.34 In this regard, understanding the immunity level of this population is essential to guiding infection prevention policies. We recently investigated the COVID-19 vaccination rates in individuals hospitalized with chronic medical conditions, which are important risk factors for severe COVID-19 (unpublished data). We found that these patients, especially those with cancer, had lower vaccination rates than the general population of a similar age. Since this was a single-center study, we aimed to determine whether there is a similar pattern at the national level.
We combined the national healthcare claims database operated by the National Health Insurance Service of Korea with the national immunization registry operated by the Korea Disease Control and Prevention Agency. South Korea has universal, single-payer healthcare coverage for all residents. Categories of chronic medical conditions to be examined were selected as previously described elsewhere.5 In brief, the diseases of interest with reports of possible association with SARS-CoV-2 in previous epidemiologic studies and those with theoretical concerns for increased risk were selected from the list of International Classification of Diseases and Related Health Problems, 10th edition (ICD-10) mapping tree (Supplementary Table 1). Patients aged ≥ 18 years who visited outpatient clinics at least thrice or were hospitalized at least once with the abovementioned conditions from March 2020 to February 2022 were included in the study. Domestic and overseas vaccination data up to May 31, 2022, were extracted. The vaccine products included in this study are Vaxzevria® (Oxford-AstraZeneca), Comirnaty® (Pfizer-BioNTech), Spikevax® (Moderna), Nuvaxovid® (Novavax), and Jcovden® (Janssen). Jcovden® as a first dose was considered equivalent to 2 doses of other vaccines. The corresponding statistics up to May 31, 2022, in the general population aged ≥ 18 years were used as comparators.6 Patients who died before February 28, 2022, when national immunization virtually ended,7 were excluded from the study to minimize immortal time bias.
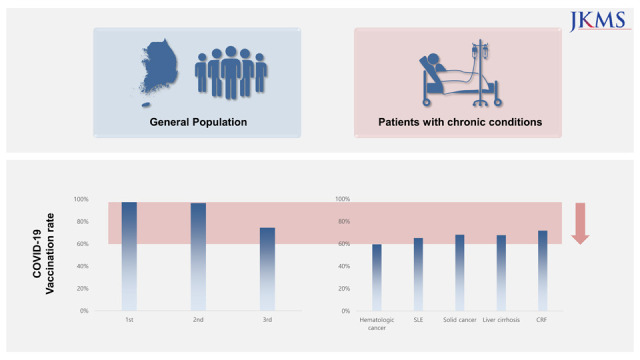
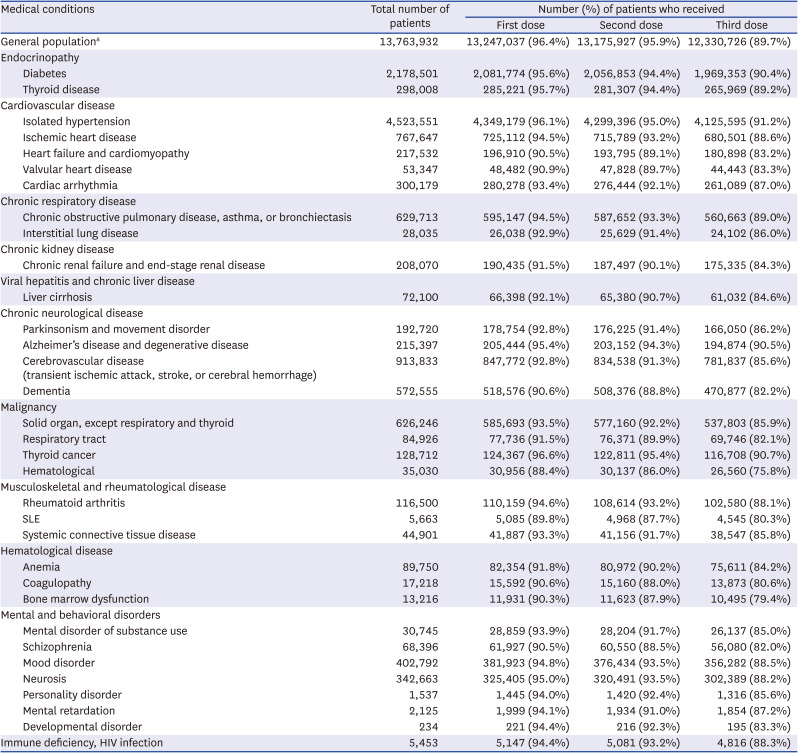
The number of patients according to the disease category and vaccination status are presented In Tables 1, 2, and 3. Among the general population, the percentages of those who received at least one dose, at least two doses, and at least 3 doses of a COVID-19 vaccine were 97.3%, 96.5%, and 74.5%, respectively. When divided by age, the third vaccination rate showed a significant difference between age groups (age 60+ years, 89.7%; age 40–59 years, 74.9%; age 18–39 years, 59.1%), in contrast to the first and second vaccination rates.
Several conditions, such as hematological cancer and systemic lupus erythematosus, were associated with vaccination rates lower by ≥ 10% than those of the national statistics in all age groups (Tables 1-3). Patients with solid cancers (except thyroid cancer), liver cirrhosis, and chronic renal failure, had lower vaccination rates in all age groups, with greater differences in the younger age groups. Low vaccine acceptance was more prominent in patients with serious and debilitating diseases, such as malignancy, than in those with less serious conditions, such as isolated hypertension. This trend was consistent with our unpublished single-institution data, which revealed lower vaccination rates in patients with malignancies. Among individuals aged ≥ 60 years, patients with heart failure, cerebrovascular disease, and dementia had lower vaccination rates than the general population of a similar age (Table 3). In some disease categories, the second and third vaccination rates were far lower in chronically ill patients than in the general population, suggesting that a considerable number actually did not complete the primary series.
There are several reasons why patients with chronic medical conditions have low acceptance of COVID-19 vaccination, such as safety concerns, incompatibility with their diseases or treatments, and lack of information.8910 Indeed, there are only a few studies on vaccine efficacy and safety in chronically ill patients. Despite this limitation, public health authorities and academic societies recommend these patients to be vaccinated based on their higher risk of severe and critical COVID-19 and no reports of major safety signals specific to them.111213141516 Additionally, while patients with comorbidities develop lower levels of immunity than people without comorbidities after the primary series,171819 a third dose significantly increased neutralizing antibody titers.2021 On the other hand, some studies revealed that patients with chronic medical conditions were more willing to accept COVID-19 vaccination.2223 Indeed, patients with certain conditions, such as isolated hypertension, that usually do not require intensive treatment, had high vaccination rates. The reasons why patients with serious comorbidities are not vaccinated might have been difficulty in accessing vaccination centers due to treatment schedules or poor conditions. In addition, younger individuals were found to be more vaccine-hesitant in previous studies.2425 This may be due to lower perceived risk of serious illness than that in older individuals or easier access to misinformation that is widespread online. For patients with hematologic diseases, there might have been more specific concerns such as vaccine-induced immune thrombotic thrombocytopenia.
Undoubtedly, public health authorities and healthcare providers should try to raise vaccination rates in these populations as much as possible. Considering the above findings, we suggest several possible ways to do this. For those who wish to be vaccinated but do not have access to vaccination centers, encouraging vaccinations at secondary and tertiary referral centers would be helpful. For example, physicians can check immunization records and administer catch-up vaccinations at the end of acute care. For those who are hesitant, it is essential to provide accurate information regarding COVID-19 and its vaccines and to encourage vaccination. It is physicians who know best about the potential benefits and risks of vaccination related to their patients’ diseases and treatments and thus can be trusted the most. In addition, poor immunogenicity is an important factor for low vaccine uptake in patients with severe immunocompromising conditions such as hematological malignancy and solid organ transplantation. Thus, physicians should also actively consider using tixagevimab–cilgavimab, a prophylactic anti-SARS-CoV-2 monoclonal antibody formulation, that will soon be introduced for these high-risk patients. Amid another pandemic of misinformation, we believe that doctors’ efforts can improve vaccination rates and maintain patient safety.
This study has several limitations. First, it was based on health insurance claims data; therefore, it was impossible to obtain detailed information on the severity of chronic medical conditions. Moreover, given that health insurance claims data are collected based on diagnostic codes, there might be a possibility of missing information or information bias. However, categories of serious and/or rare diseases, such as malignancies, are reliable, as diagnoses are verified for reimbursement. Second, although this data might be outdated at the time of submission and publication, we believe it is still relevant since the national statistics did not significantly change since March 1, 2022. Third, natural SARS-CoV-2 infections, by which people could develop protective immunity, were not considered in this study, leaving the possibility that people may have higher immunity levels than those assessed using vaccination data alone.
In conclusion, there is a considerable gap between the COVID-19 vaccination rates among patients with chronic medical conditions and those of the general population, especially in younger individuals. Public health authorities and healthcare providers should be aware of this and try to vaccinate these patients to avoid preventable morbidity and mortality.
ACKNOWLEDGMENTS
This study used the database of the Korea Disease Control and Prevention Agency and the National Health Insurance Service of Korea for policy and academic research. The research number of this study is KDCA-NHIS-2022-1-448.
Notes
Author Contributions:
Conceptualization: Cheong HJ.
Data curation: Kim YE, Kim DW, Jang HY.
Investigation: Nham E, Kim YE, Jumg J, Kim DW, Jang H, Hyun H, Seong H, Yoon JG, Noh JY, Song JY, Kim WJ, Cheong HJ.
Methodology: Nham E, Kim YE, Jung J, Kim DW, Jang H.
Software: Kim YE, Jumg J, Kim DW, Jang H.
Validation: Nham E, Kim YE, Jung J, Kim DW, Jang H.
Writing - original draft: Nham E.
Writing - review & editing: Nham E, Kim YE, Jung J, Hyun H, Seong H, Yoon JG, Noh JY, Song JY, Kim WJ, Cheong HJ.
References
1. Kim JY, Choe PG, Oh Y, Oh KJ, Kim J, Park SJ, et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. 2020; 35(5):e61. PMID: 32030925.
2. Expansion of indication for second booster for prevention of COVID-19 resurgence and call for vaccination (July 13). Updated 2022. Accessed July 16, 2022.
http://ncov.mohw.go.kr/tcmBoardView.do?brdId=3&brdGubun=31&dataGubun=&ncvContSeq=6752&contSeq=6752&board_id=312&gubun=ALL
.
3. Jung J, Lee J, Jo S, Bae S, Kim JY, Cha HH, et al. Nosocomial outbreak of COVID-19 in a hematologic ward. Infect Chemother. 2021; 53(2):332–341. PMID: 34216126.
4. Sung HK, Kim JY, Heo J, Seo H, Jang YS, Kim H, et al. Clinical course and outcomes of 3,060 patients with coronavirus disease 2019 in Korea, January-May 2020. J Korean Med Sci. 2020; 35(30):e280. PMID: 32743995.
5. Ji W, Huh K, Kang M, Hong J, Bae GH, Lee R, et al. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: a nationwide case-control study. J Korean Med Sci. 2020; 35(25):e237. PMID: 32597048.
6. Current status of COVID-19 outbreak and vaccination in Korea. Updated 2022. Accessed June 15, 2022.
http://ncov.mohw.go.kr/tcmBoardView.do?brdId=3&brdGubun=31&dataGubun=&ncvContSeq=6536&contSeq=6536&board_id=312&gubun=BDJ
.
7. Coronavirus disease-19 (COVID-19), Republic of Korea: press release. Updated 2022. Accessed July 18, 2022.
http://ncov.mohw.go.kr/tcmBoardList.do?brdId=&brdGubun=&dataGubun=&ncvContSeq=&contSeq=&board_id=140&gubun=
.
8. Chun JY, Kim SI, Park EY, Park SY, Koh SJ, Cha Y, et al. Cancer patients’ willingness to take COVID-19 Vaccination: a nationwide multicenter survey in Korea. Cancers (Basel). 2021; 13(15):3883. PMID: 34359783.
9. Moujaess E, Zeid NB, Samaha R, Sawan J, Kourie H, Labaki C, et al. Perceptions of the COVID-19 vaccine among patients with cancer: a single-institution survey. Future Oncol. 2021; 17(31):4071–4079. PMID: 34337969.
10. Chan WL, Ho YT, Wong CK, Choi HC, Lam KO, Yuen KK, et al. Acceptance of COVID-19 vaccination in cancer patients in Hong Kong: approaches to improve the vaccination rate. Vaccines (Basel). 2021; 9(7):792. PMID: 34358208.
11. COVID-19 vaccines for people who are moderately or severely immunocompromised. Updated April 12, 2022. Accessed April 27, 2022.
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html
.
12. COVID-19 advice for the public: getting vaccinated. Updated April 13, 2022. Accessed April 27, 2022.
https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice
.
13. AASLD Expert Panel Consensus Statement. Vaccines to prevent COVID-19 in patients with liver disease. Updated Mar 28, 2022. Accessed Jul 10, 2022.
https://www.aasld.org/covid-19-and-liver#statement-on-covid-19-vaccines-and-clinical-best-practice-for-hepatology-and-liver-transplant-providers
.
14. American Heart Association. Questions about COVID-19 vaccination. Updated January 12, 2022. Accessed July 10, 2022.
https://www.heart.org/en/coronavirus/coronavirus-questions/questions-about-covid-19-vaccination
.
15. National Kidney Foundation. Health guidance for kidney patients. Updated June 3, 2022. Accessed July 10, 2022.
https://www.kidney.org/covid-19
.
16. ASH-ASTCT COVID-19 vaccination for HCT and CAR T cell recipients: frequently asked questions. Updated March 22, 2022. Accessed April 27, 2022.
https://www.hematology.org/covid-19/ash-astct-covid-19-vaccination-for-hct-and-car-t-cell-recipients
.
17. Mair MJ, Berger JM, Berghoff AS, Starzer AM, Ortmayr G, Puhr HC, et al. Humoral immune response in hematooncological patients and health care workers who received SARS-CoV-2 vaccinations. JAMA Oncol. 2022; 8(1):106–113. PMID: 34591965.
18. Espi M, Charmetant X, Barba T, Koppe L, Pelletier C, Kalbacher E, et al. The ROMANOV study found impaired humoral and cellular immune responses to SARS-CoV-2 mRNA vaccine in virus-unexposed patients receiving maintenance hemodialysis. Kidney Int. 2021; 100(4):928–936. PMID: 34284044.
19. Oosting SF, van der Veldt AA, GeurtsvanKessel CH, Fehrmann RS, van Binnendijk RS, Dingemans AC, et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021; 22(12):1681–1691. PMID: 34767759.
20. Mair MJ, Berger JM, Mitterer M, Gansterer M, Bathke AC, Trutschnig W, et al. Third dose of SARS-CoV-2 vaccination in hemato-oncological patients and health care workers: immune responses and adverse events - a retrospective cohort study. Eur J Cancer. 2022; 165:184–194. PMID: 35248840.
21. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021; 385(7):661–662. PMID: 34161700.
22. Williams L, Gallant AJ, Rasmussen S, Brown Nicholls LA, Cogan N, Deakin K, et al. Towards intervention development to increase the uptake of COVID-19 vaccination among those at high risk: outlining evidence-based and theoretically informed future intervention content. Br J Health Psychol. 2020; 25(4):1039–1054. PMID: 32889759.
23. Aw J, Seng JJ, Seah SS, Low LL. COVID-19 vaccine hesitancy-A scoping review of literature in high-income countries. Vaccines (Basel). 2021; 9(8):900. PMID: 34452026.
24. Mondal P, Sinharoy A, Su L. Sociodemographic predictors of COVID-19 vaccine acceptance: a nationwide US-based survey study. Public Health. 2021; 198:252–259. PMID: 34492505.
25. Diesel J, Sterrett N, Dasgupta S, Kriss JL, Barry V, Vanden Esschert K, et al. COVID-19 vaccination coverage among adults - United States, December 14, 2020-May 22, 2021. MMWR Morb Mortal Wkly Rep. 2021; 70(25):922–927. PMID: 34166331.
Table 1
Coronavirus disease 2019 vaccination rates in patients aged 18–39 years with various chronic medical conditions
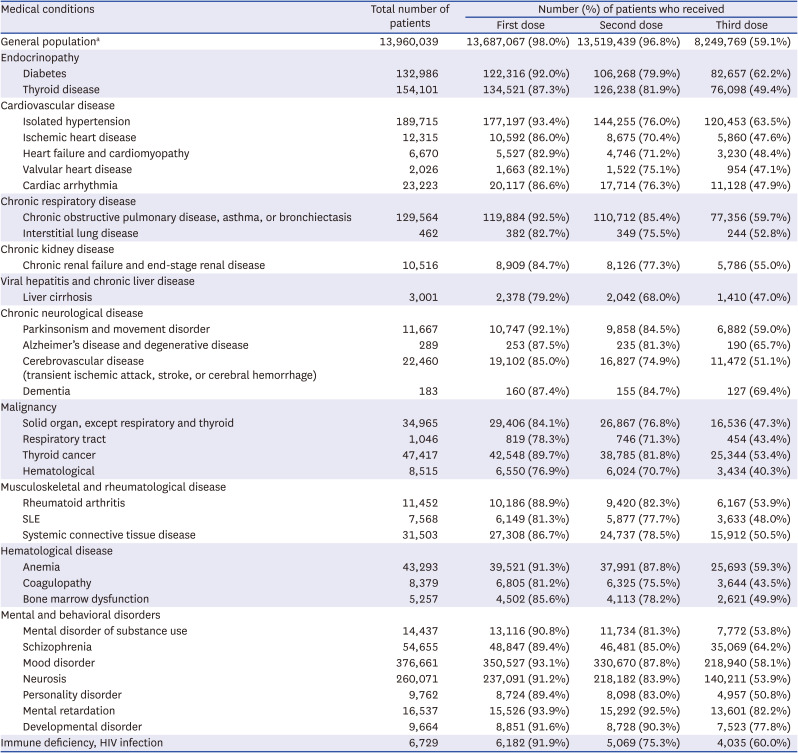
Table 2
Coronavirus disease 2019 vaccination rates in patients aged 40–59 years with various chronic medical conditions
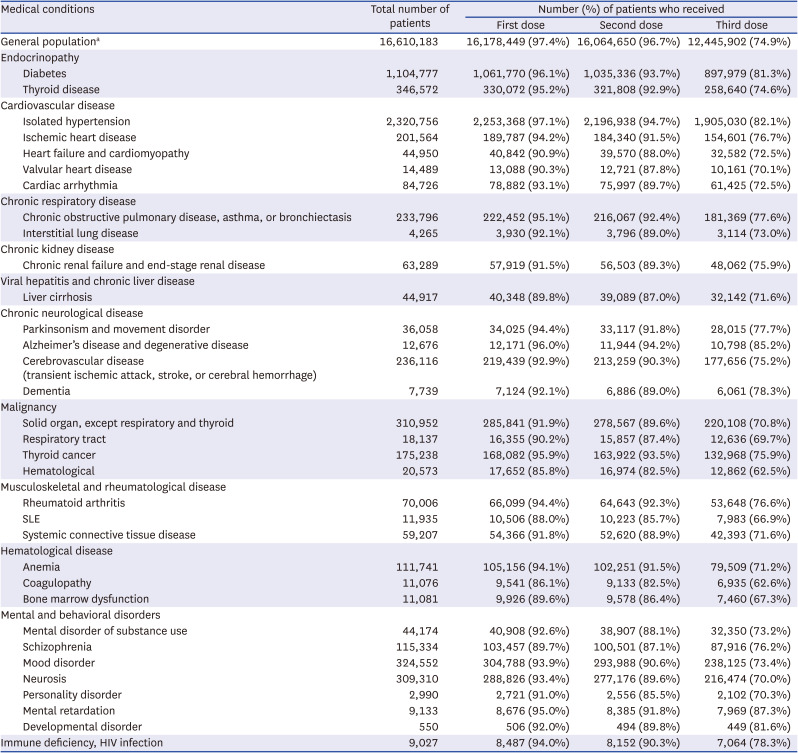
Table 3
Coronavirus disease 2019 vaccination rates in patients aged 60–120 years with various chronic medical conditions




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download