I. Introduction
Surgical extraction of the third molar is a common procedure performed in oral and maxillofacial surgery; however, it can cause extreme stress in patients who experience fear and anxiety about dental treatment. Sedation is useful, as it can reduce the fear of tooth extraction and increase satisfaction with dental treatment
1.
Of various sedative agents, midazolam (MDZ) and dexmedetomidine (DEX) are used frequently
2,3. MDZ is a derivative of benzodiazepine with sedative, anti-anxiety, muscle relaxant, and anticonvulsant properties. It promotes cardiopulmonary stability, has multiple routes of administration and a short half-life, induces antecedent memory loss, and results in good patient satisfaction
1,4. DEX exhibits effective sedative, analgesic, and anxiolytic effects with minimal respiratory system suppression
4. In addition, it has a sympatholytic effect that can relieve tachycardia and hypertension
5.
Studies comparing the efficacy of MDZ and DEX in the field of oral and maxillofacial surgery have been actively conducted; however, numerous biases are present, and studies comparing the aspect of cost are rare
6. Therefore, the purpose of this study was to investigate differences in the sedative effects of MDZ and DEX in patients undergoing surgical extraction of the mandibular third molar. The investigators hypothesized that DEX would be advantageous compared to MDZ in terms of vital sign stability despite the relatively high price. The specific aim of the study was to compare the efficacy of the two agents with consideration of vital sign stability and cost.
III. Results
Among the 185 patients, 103 received MDZ and 82 received DEX. The mean age was 27.34±9.15 years (range, 13-60 years). Most patients were healthy without any systemic diseases. There were 9 cases of diabetes mellitus or hypertension, 1 case of stable angina, 1 case of epilepsy, and 1 case of chronic myeloid leukemia. All of these were well-controlled. The SpO
2 was maintained above 95 in all patients, and there were no cases of respiratory failure or bradycardia. No reversal agent was administered due to over-sedation when using MDZ, and no atropine was used for DEX.
Table 1 summarizes the comparison of the covariates in the two groups before and after PSM. Before PSM, there was a statistically significant difference in age and Pederson scale between the two groups; however, this was corrected through PSM.
Supplementary Table 1 summarizes the correlation between the covariates and vital signs. All covariates except for the sleeping time ratio affected vital signs. Therefore, to study vital signs only by sedative difference, the covariates of the two groups had to be corrected, and for this purpose, PSM was used.
Table 2 summarizes the comparison of outcome variables according to sedative differences. Post-PSM data showed that the frequency of vital sign outliers changed by more than 30% compared to the initial value, which was 49.3% in the MDZ group and 22.6% in the DEX group for HR. However, no patients required cardiac care due to this change. HR showed a higher value in the MDZ group during all periods of sedative administration.(
Fig. 1. A) SBP was higher in the MDZ group 20 minutes after local anesthesia and at the beginning of the primary closure.(
Fig. 1. B) DBP was higher in the DEX group 5 minutes after sedative administration and in the MDZ group at the beginning of the primary closure.(
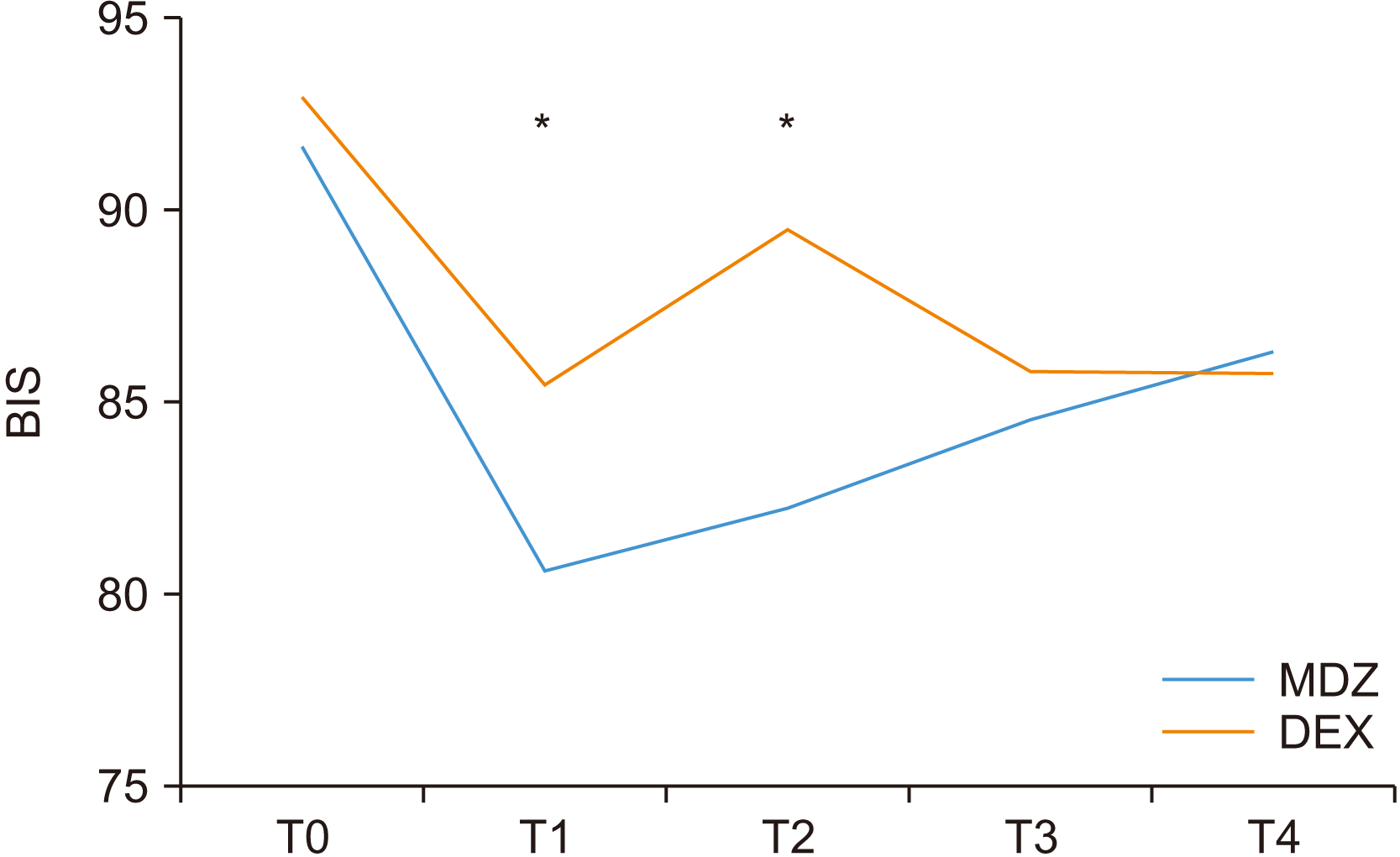
Fig. 1. C) As for the average cost of sedatives per patient, the MDZ group had a much lower cost. There was no significant difference in the OAAS, level of amnesia, or patient satisfaction between the two groups. The BIS showed a lower value in the MDZ group 5 minutes after sedative administration and at the time of local anesthesia.(
Fig. 2)
Supplementary Tables 2 and 3 summarize the comparison of values according to each time interval in each group.
IV. Discussion
Since MDZ and DEX are the most commonly used sedatives, it is of clinical value to compare the sedative effect, vital sign stability, and cost of the two agents. The purpose of this study was to compare the differences between the two drugs, focusing on these points. In this study, the sedative effect assessed by the surgeon and the patient was similar between the two groups. DEX was advantageous over MDZ in vital sign stability; however, this difference did not have clinical significance in relatively healthy patients within ASA II. In terms of cost, MDZ was advantageous compared with DEX.
Demographic differences among patients can affect differences in the degree of dental anxiety. Acharya et al.
12 and Udoye et al.
13 have reported significant differences in dental phobia according to sex and age, using the DAS questionnaire. Similar results were also reported in a study by Appukuttan et al.
14, which used the Tamil version of the modified DAS questionnaire. In addition, since dental anxiety can affect the hemodynamic vital signs
15, differences in dental anxiety may cause errors in the analysis of vital sign-related effects of sedative agents. However, in this study, no difference in dental anxiety was found between the two groups. This study was retrospective in nature; however, we aimed to reduce study bias as much as possible by correcting for dental anxiety and covariates, such as sex and age, through the PSM method. Therefore, differences in the hemodynamic signs observed in this study were mainly due to differences between the two drugs themselves.
Representative reactions of DEX on the cardiovascular system are bradycardia and lowering of blood pressure. Since these reactions can be controlled by using an appropriate infusion rate, DEX can be used as a sedative agent in patients with ischemic heart disease
16. In addition, this characteristic of DEX acts as an advantage in reducing blood loss during surgery and securing a field of view in the surgical field
17. In this study, HR and SBP decreased more, and the amount of change was smaller for DEX compared with MDZ. Thus, it was possible to confirm the clinical stability of DEX from a hemodynamic point of view. In many previous studies, the clinical hemodynamic stability of DEX compared with that of MDZ has been supported. In a study conducted by Yu et al.
18 comparing the SpO
2 and hemodynamic effects of the MDZ/fentanyl combination and DEX/fentanyl combination during sedation for surgical extraction, the HR was lower in the DEX/fentanyl group. Barends et al.
19 reported that MDZ could cause unwanted hypertension during surgery. In addition, in a randomized double-blinded study comparing the sedation effects of DEX and the MDZ/fentanyl combination, the mean arterial pressure and HR were lower in the DEX group
20. However, unlike these study results, in a study comparing the sedation effect of MDZ and DEX in elderly patients undergoing spinal anesthesia, the mean arterial pressure during the intraoperative 120 minutes was higher with DEX than with MDZ
21. Therefore, medical staff performing sedation must be prepared for such exceptional cases.
In this study, the cost of the two agents were significantly different, with MDZ costing much less. In a study comparing the cost-effectiveness of MDZ and DEX in patients undergoing mechanical ventilation in an intensive care unit environment, Lachaine and Beauchemin
22 also reported that MDZ was cheaper than DEX in terms of price itself. However, they concluded that DEX was more economical when considering the cost of managing mechanical ventilation and delirium
22. In addition, in another study comparing the economic feasibility of MDZ, DEX, and propofol in an intensive care setting, DEX was reported to be the least expensive
23.
Sedation agents have been compared for their vital sign stability and cost as well as various other variables, and each study has shown slightly different results. In this study, there was no statistically significant difference in the OAAS or satisfaction in the two groups. However, Wang et al.
24 reported that the OAAS value was higher in the MDZ group than in the DEX group. In addition, in a systematic review comparing the efficacy and safety of MDZ and DEX, the DEX group had higher satisfaction for patients and clinicians than the MDZ group
19. In this study, the BIS value was lower 5 minutes after sedative administration and during local anesthesia in the MDZ group than in the DEX group. However, Fan et al.
2 reported that there was no statistically significant difference in the BIS between the two groups.
The demographic data, such as the patient’s sex and age, and various factors that can affect the sedative effect, such as BMI, sleep time, fear of dental treatment, and type and difficulty of the surgery, were controlled for in this study. Thus, this study has the strength of objectively evaluating the effects of the sedatives themselves and the stability of the vital signs with relatively little bias. In addition, the efficacy of the drugs was compared in consideration of the cost, which has not been addressed well in previous studies. To the best of our knowledge, this study is the first paper to compare the cost-effectiveness of sedation agents during the extraction of third molars under intravenous sedation. However, this study has several limitations. First, it has an inherent limitation as a retrospective nonrandomized concurrent cohort study, despite the effort to correct bias through PSM. Second, the dosing of MDZ and DEX is different for each study, and this might act as a study bias. Third, unlike DEX, MDZ does not have an analgesic effect by itself; hence, pethidine was used in combination with MDZ to reflect the characteristics of these drugs, which could act as a confounder. Fourth, the respiratory rate and capnography were not monitored. Fifth, this was an analysis of the state during sedation, and the recovery after sedation was not analyzed. It is necessary to evaluate the respiratory rate and capnography during sedation and the degree of recovery after sedation through prospective studies in the future.
V. Conclusion
The sedative effects of MDZ and DEX were similar in this study, and the surgeons and patients were relatively satisfied. Given the difference in vital sign stability and cost, the indications for the use of MDZ or DEX during extraction of third molars under intravenous moderate sedation can be summarized as follows. First, the use of MDZ is recommended for patients who are generally relatively healthy, considering the cost. Second, in patients with systemic diseases with cardiac and hemodynamic instability, such as uncontrolled hypertension or myocardial infarction, the use of DEX, which has more favorable vital sign stability, is recommended, despite the high cost.
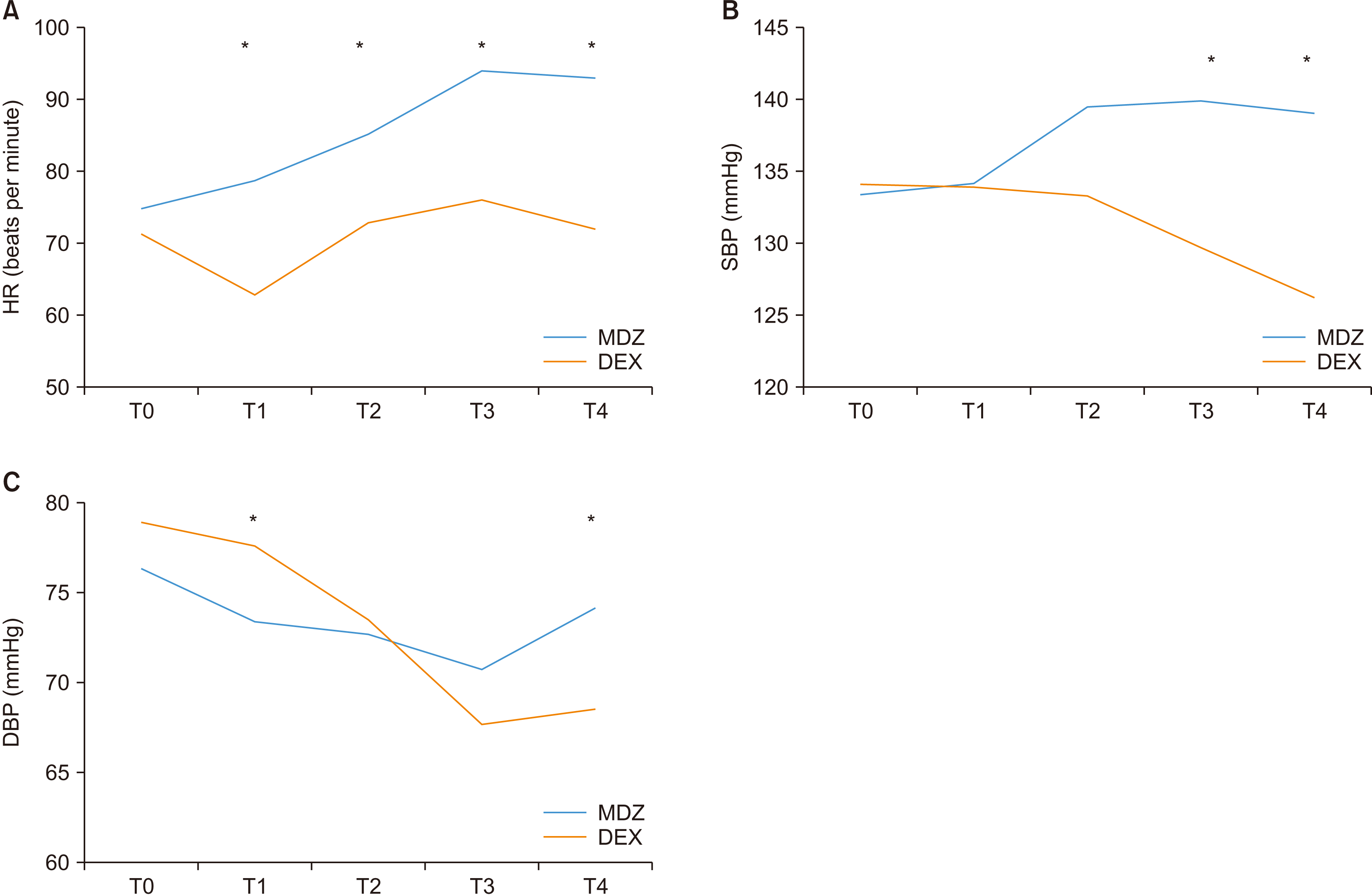




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download