Abstract
Background
Enteral nutrition (EN) supply within 48 hours after intensive care unit (ICU) admission improves clinical outcomes. The “new ICU evaluation & development of nutritional support protocol (NICE-NST)” was introduced in an ICU of tertiary academic hospital. This study showed that early EN through protocolized nutritional support would supply more nutrition to improve clinical outcomes.
Methods
This study screened 170 patients and 62 patients were finally enrolled; patients who were supplied nutrition without the protocol were classified as the control group (n=40), while those who were supplied according to the protocol were classified as the test group (n=22).
Results
In the test group, EN started significantly earlier (3.7±0.4 days vs. 2.4±0.5 days, P=0.010). EN calorie (4.0±1.0 kcal/kg vs. 6.7±0.9 kcal/kg, P=0.006) and protein (0.17±0.04 g/kg vs. 0.32±0.04 g/kg, P=0.002) supplied were significantly higher in the test group. Although EN was supplied through continuous feeding in the test group, there was no difference in complications such as feeding hold due to excessive gastric residual volume or vomit, and hyper- or hypo-glycemia between the two groups. Hospital mortality was significantly lower in the group that started EN within 1.5 days (42.9% vs. 11.8%, P=0.018). The proportion of patients who started EN within 1.5 days was higher in the test group (40.9% vs. 17.5%, P=0.044).
Nutrition support means supplying calories, proteins, electrolytes, fluids, etc., through enteral nutrition (EN) or parenteral nutrition (PN). Nutrition in critically ill patients has several clinical guidelines. Some recommendations are significantly different in these guidelines, reflecting a lower level of evidence [1-6]. The benefits of early EN (within 24 to 48 hours after intensive care unit [ICU] admission) in improving clinical outcomes are well known [4,7-15]. Meta-analysis of 21 randomized control trials by Taylor et al. [4] showed that the early EN group had a significant decrease in mortality (odds ratio [OR], 0.70; 95% confidence interval [CI], 0.49–1.00) and infection complications (OR, 0.47; 95% CI, 0.58–0.93). However, the amount of EN supplied showed no difference in clinical outcomes. The EDEN trial showed no significant difference in infection complications such as ventilator-associated pneumonia (P=0.72) and 60-day mortality (P=0.77) between the trophic feeding group supplied fixed calorie with 400 kcal per day and the full enteral feeding group for the first 6 days after ICU admission [16]. Marik et al. [17] reported no significant difference in hospital mortality (OR, 0.91; 95% CI, 0.75–1.11) and infection complications (OR, 1.03; 95% CI, 0.84–1.27) in a meta-analysis of six studies that compared normo-caloric feeding (72%–77% of goal) with trophic feeding or permissive underfeeding (20% or 49% of goal).
This study aimed to determine whether protocolized nutrition support for ICU patients could improve EN feeding and clinical outcomes. In addition, the risk of complications such as hold of feeding due to excessive gastric residual volume (GRV) or vomiting, and hyperglycemia or hypoglycemia events when continuous feeding and GRV check protocol were applied to the nutrition support protocol was evaluated.
This study is a single-institution retrospective cohort study of a new ICU evaluation & development of nutritional support protocol (NICE-NST) protocol, introduced for medical ICU patients in a tertiary academic hospital from December, 2018. This study was conducted in accordance with the amended Declaration of Helsinki and was approved by the Institutional Review Board of Seoul National University Bundang Hospital where the study was conducted (No. B-1907-550-107). Patient consent was waived because the NICE-NST protocol was implemented as an in-hospital quality improvement project, and this study was a retrospective review of the outcomes.
The contents of NICE-NST protocol were as follows: (1) the Penn state equation was used to determine target caloric requirements [18], which was similar to the indirect calorimetry value. (2) Patients were categorized into group 1 (<5 scores, with abnormal liver function, or with abnormal renal function), and group 2 (>5 scores or <70% of the daily nutrients requirement target after 7 days of ICU day) using nutrition risk in critically ill (NUTRIC) score. Group 1 nutrition was by EN only, but group 2 nutrition was by supplementary PN with EN to meet nutritional targets. Both groups targeted 100% of the required daily calories and 1.3 g/kg/day of protein (Table 1). EN supply was through continuous feeding with tube or gastrostomy for 16 hours from 9 AM to 1 AM the next day. The GRV was measured at 9 AM, 5 PM, and 11 PM, considering the ICU nurse-working schedule. If GRV was > 250 ml, EN feeding is held, and prokinetics are given thrice daily. After 2 hours of withholding EN feeding, GRV was rechecked, and the same protocol was repeated until 1 AM the next day (Figure 1).
The group that was supplied nutrition without NICE-NST protocol was the “control” group, and the group that was supplied nutrition with NICE-NST protocol was the “test” group. Patients admitted to medical ICU from December 2017 to March 2018 and other patients admitted to medical ICU from December 2018 to March 2019 were screened as the control and test groups, respectively. Exclusion criteria include ICU discharge within one day, non-tube feeding, transfer to medical ICU from other ICU, nutrition support team consultation before ICU admission, continuous renal replacement therapy (CRRT) application within 5 days, intermittent hemodialysis (HD) before ICU admission, and long-term inpatients in medical ICU because of legal problem.
Supplying nutrition with protocol in ICU will ensure multidisciplinary discussions on nutrient supply; it will provide appropriate calorie and other nutrients to critically ill patients quickly. Therefore, we expect that the start and amount of EN will significantly improve by following the NICE-NST protocol. In addition, it is expected to improve the clinical outcomes such as ICU mortality, hospital mortality, duration of ICU admission, etc.
Age, sex, cause of ICU admission, and medical history data were collected to evaluate baseline characteristics. Initial arterial oxygen pressure divided by the fraction of inspired oxygen (P/F ratio), Acute Physiology And Chronic Health Evaluation (APACHE) II score, nutrition severity, and NUTRIC score were collected to assess disease severity at the time of ICU admission. Daily body weight, daily calorie supply of dextrose for main fluid, daily calorie and protein supply of EN, and daily calorie and protein supply of PN data during the first 5 days in ICU were collected to evaluate the primary calorie and protein supply. The total amount of calories and protein supplied throughout one day was calculated with administered total daily volume for each item. For example, suppose EN feeding in which 1 kcal per 1 ml and 0.05 g of protein per 1 ml were supplied with 32 ml per hour for 10 hours. In that case, the total calorie supply of EN feeding will be 320 kcal, and the total protein supply of EN feeding will be 16 g. The total calorie was calculated as the sum of EN calorie supply, PN supply, and supplied dextrose and albumin. Total protein was calculated as the sum of protein of EN, PN, and albumin supplied.
In addition, in order to analyze feeding side effects, data on the total daily count of EN feeding hold due to GRV or vomit, the ratio of hyperglycemia (blood glucose level >250 mg/dl) events to total daily glucose check counts, the ratio of hypoglycemia (blood glucose level <70 mg/dl) events to total daily glucose check counts, and the total amount of daily steroid use were collected. Because the total number of blood glucose checks was different according to the status of patients, a ratio was used to analyze hyperglycemia and hypoglycemia events. In addition, the total amount of daily steroid can also affect blood glucose level; therefore, the total amount of daily steroid use was analyzed.
Fisher's exact test and Pearson Chi-square were used for the comparison of categorical variables. Mann-Whitney test and Student t-test were used for the analysis of continuous variables. We used repeated-measures analysis of variance (RM-ANOVA) to analyze repeated-measured variables. Logistic regression for ICU mortality was performed with age, sex, body mass index (BMI), nutrition severity, the time duration from admission in ICU to the start of EN feeding (Admission to EN time), and mean amount of supplied EN calorie (kcal/kg) or protein (g/kg) for 5 days. The results were considered significant, with P-values less than 0.05. All analyses were conducted using the IBM SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
This study screened 170 patients. One patient discharged from the medical ICU within one day was excluded. Two patients who were given soft bland diet without tube feeding were excluded. Because the NICE-NST protocol was applied only to the medical ICU patients, 31 patients transferred from the other ICU, such as surgical or emergency ICU, were excluded. Since the NICE-NST protocol was applied at the time of medical ICU admission, four patients whose nutrition support team consultation was conducted before entering the medical ICU were excluded. Because diverse solvents are used in dialysis and protein loss is inevitable, the amount of calorie and protein supply could not be confirmed exactly in CRRT or HD. Thus, 45 patients who had been applied CRRT within 5 days from medical ICU admission and 4 patients who had previously received intermittent HD were excluded. In addition, three patients hospitalized for a long time in medical ICU because of legal problems were excluded. Initiating EN within at least 48 hours of ICU admission was described as early EN in previous studies [4,7-15]. Since one of the aims of this study was to determine whether early EN was achieved when the protocol was applied, only patients whose protocol application time was at least 2 days were selected and analyzed. In addition, because a feeding protocol was provided through nutrition support team consultation, only patients with the duration from admission in ICU to nutrition support team consultation (Admission to nutrition support team time) of less than 2 days were included in the test group. Finally, the data of remaining 62 patients (40 patients in the control group, 22 in the test group) were analyzed (Figure 2).
Baseline characteristics showed no difference in age, sex, BMI, underlying diseases, and the cause of admission to ICU between the two groups. Furthermore, there was no difference in disease severity, such as APACHE II score, initial P/F ratio, and the amount of steroid use converted to that of methylprednisolone (Supplementary Table 1).
The calorie and protein supply, which are the primary outcomes of this study, showed that total calorie and protein supplied were not significantly different between the two groups. However, the supply of EN calorie (4.0±1.0 kcal/kg vs. 6.7±0.9 kcal/kg, P=0.006) and protein (0.17±0.04 g/kg vs. 0.32±0.04 g/kg, P=0.002) were significantly higher in the test groups (Table 2). Further, the Admission to EN time was significantly shorter in the test groups (3.7±0.4 days vs. 2.4±0.5 days, P=0.010) (Table 3). The trends of supplying calories and protein over time are shown in Figure 3. EN calorie and protein supply analyzed by RM-ANOVA were significantly higher in the test group. However, during the early stage of ICU admission, more calories and protein were supplied in the control group. There was no difference in serum albumin level change between admission in ICU and 7 days after admission. No difference was observed in the number of feeding hold due to excessive GRV or vomiting during 5 days in ICU and the ratio of hyperglycemia events to total daily glucose check counts or the ratio of hypoglycemia events to total daily glucose check counts during 5 days in ICU (Table 3). There was no difference in ICU mortality, hospital mortality, and the time duration from admission to discharge in ICU (ICU duration) between the two groups (Table 3). The average admission to EN time of the test group exceeded 2 days (2.4±0.5 days) (Table 3).
Additional analysis was performed with 80 patients regardless of applying NICE-NST protocol (Supplementary Figure 1). The hospital mortality was significantly lower in the group that started EN feeding within 1.5 days (42.9% vs. 11.8%, P=0.018), and the ICU mortality was lower in the same group with marginal significance (28.6% vs. 5.9%, P=0.051) (Table 4). APACHE II score (32.3±0.96 vs 31.9±1.90, P=0.898) and initial P/F ratio (200.1±14.97 vs. 215.0±32.16, P=0.705) were not significantly different regardless of starting EN feeding within 1.5 days or not. On the other hand, there were significantly more patients with admission to EN time less than 1.5 days in the test group than in the control group (17.5% vs. 40.9%, P=0.044; OR, 3.26; 95% CI, 1.005–10.600).
Multivariable logistic regression was conducted to confirm whether mortality was associated with the amount of EN feeding. The mean EN calorie (OR, 0.815; 95% CI, 0.681–0.975) (Supplementary Table 2) and protein (OR, 0.017; 95% CI, 0.000–0.696) (Supplementary Table 3) per weight were inversely correlated with ICU mortality. Finally, when patients were divided according to 5 kcal/kg of EN calorie supply or 0.2 g/kg of EN protein supply, ICU mortality was significantly lower in the group supplied more than 5 kcal/kg of EN calorie or 0.2 g/kg of EN protein (7.7% vs. 34.8%; OR, 6.400; 95% CI, 1.338–30.606). Hospital mortality was not significant but tended to be lower in the same group (23.1% vs. 45.7%; OR, 2.800; 95% CI, 0.950–8.255) (Supplementary Table 4).
It is well known that early EN feeding within 24 to 48 hours had several clinical benefits, such as reducing mortality significantly [4,7-15]. In this present study, it is expected that EN feeding will commence earlier and supply more nutrition without significant EN-related complications using the NICE-NST protocol. In addition, the clinical outcomes such as ICU mortality and hospital mortality were expected to improve. This study showed that the amount of calories and protein supplied through EN was significantly higher in the test group that used the NICE-NST protocol. However, no significant difference was observed in total calorie and protein supply between the two groups, which means that the supply through PN was higher in the control group. These results showed that an effective increase in EN feeding might not be achieved without nutrition protocol, increasing the nutrition supply through PN rather than EN. In addition, since EN feeding was supplied by continuously feeding for only 16 hours in the NICE-NST protocol, this result indicates that the nutrition protocol in ICU can effectively increase EN feeding within 5 days.
There was no difference in the number of feeding hold during the first 5 days in ICU due to excessive GRV or vomiting, and no difference in hyperglycemia or hypoglycemia events between the two groups. Therefore, this study confirmed that feeding for 16 hours from 9 AM to 1 AM the next day, measuring GRV three times a day, withholding EN feeding when the GRV exceeds 250 ml, and rechecking the GRV every 2 hours after feeding hold are sufficiently applicable to feeding protocol.
Taylor et al. [4] showed a significant decrease in mortality and infection complications in the early EN group within 24 to 48 hours after ICU admission. The present study showed a significantly shorter admission to EN time in the test groups (3.7±0.4 days vs. 2.4±0.5 days, P=0.010). However, ICU mortality and hospital mortality were not significantly different between the two groups. This may be because admission to EN time of the test group exceeded 2 days and a small number of patients were included in test group. When the data of 80 patients regardless of applying NICE-NST protocol were analyzed, the hospital mortality was significantly lower in the group that started EN feeding within 1.5 days, and ICU mortality was also lower in the same group with marginal significance. In addition, there were significantly more patients within 1.5 days of admission to EN time in the test group than in the control group. These results suggest that NICE-NST protocol increases the likelihood of starting EN feeding within 1.5 days, leading to improved ICU or hospital mortality.
The EDEN study showed no significant difference in 60-day mortality and complication between the trophic feeding and full enteral feeding groups until 6 days after ICU admission [16]. In this present study, the ICU mortality was significantly lower in the group supplied ≥5 kcal/kg of EN calorie or ≥0.2 g/kg of EN protein, and the hospital mortality was not significant but tended to be lower in the same group. In other words, EN supply <5 kcal/kg of EN calorie or <0.2 g/kg of EN protein, equivalent to less than trophic feeding, might increase ICU and hospital mortality.
This study has several limitations. It was a single-center and only medical ICU; therefore, the number of study subjects was small. Because it is a retrospective study, not all variables could be controlled, and biases that were not taken into account may have influenced the results. Clinical outcomes such as ICU mortality, hospital mortality, and ICU duration were analyzed with nutrition supply data for only 5 days after ICU admission; hence, variables that were not considered may have affected the results. Therefore, a randomized control trial is required to confirm the efficacy and safety of the NICE-NST protocol.
The EN calorie and protein supply in critically ill patients could be improved using the NICE-NST protocol without increased complications. The supply of EN calorie and protein within 5 days had a significant effect on improving ICU mortality.
▪ Early enteral nutrition through protocolized nutritional support may supply more nutrition to improve clinical outcomes.
▪ The “new ICU evaluation & development of nutritional support protocol (NICE-NST)” may improve enteral nutrition supply and mortality of critically ill patients without increasing complications.
ACKNOWLEDGMENTS
This work was supported by the Seoul National University Bundang Hospital Research Fund (Grant no. 06-2019-050).
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4266/acc.2022.00220.
Supplementary Table 2.
Logistic regression model for ICU mortality with EN calorie per weight
Supplementary Table 3.
Logistic regression model for ICU mortality with EN protein per weight
Supplementary Table 4.
Mortality in EN calorie below or more than 5 kcal/kg or in EN protein below or more than 0.2 g/kg
Supplementary Figure 1.
Flowchart of the study participant selection for additional analysis. The control group was supplied nutrition without protocol, while the test group was supplied nutrition with protocol. ICU: intensive care unit; CRRT: continuous renal replacement therapy; HD: hemodialysis. Admission to nutrition support team time: the time duration from admission in ICU to nutrition support team consultation.
REFERENCES
1. Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, et al. ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr. 2006; 25:210–23.

2. Dhaliwal R, Cahill N, Lemieux M, Heyland DK. The Canadian critical care nutrition guidelines in 2013: an update on current recommendations and implementation strategies. Nutr Clin Pract. 2014; 29:29–43.

3. Preiser JC, van Zanten AR, Berger MM, Biolo G, Casaer MP, Doig GS, et al. Metabolic and nutritional support of critically ill patients: consensus and controversies. Crit Care. 2015; 19:35.

4. Taylor BE, McClave SA, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit Care Med. 2016; 44:390–438.

5. Arabi YM, Casaer MP, Chapman M, Heyland DK, Ichai C, Marik PE, et al. The intensive care medicine research agenda in nutrition and metabolism. Intensive Care Med. 2017; 43:1239–56.

6. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019; 38:48–79.

7. Braga M, Gianotti L, Gentilini O, Parisi V, Salis C, Di Carlo V. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit Care Med. 2001; 29:242–8.

8. Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med. 2001; 29:2264–70.

9. Kudsk KA. Current aspects of mucosal immunology and its influence by nutrition. Am J Surg. 2002; 183:390–8.

10. Gramlich L, Kichian K, Pinilla J, Rodych NJ, Dhaliwal R, Heyland DK. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients?: a systematic review of the literature. Nutrition. 2004; 20:843–8.

11. Marik PE, Zaloga GP. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ. 2004; 328:1407.

12. Artinian V, Krayem H, DiGiovine B. Effects of early enteral feeding on the outcome of critically ill mechanically ventilated medical patients. Chest. 2006; 129:960–7.

13. Kang W, Kudsk KA. Is there evidence that the gut contributes to mucosal immunity in humans? JPEN J Parenter Enteral Nutr. 2007; 31:246–58.

14. Doig GS, Heighes PT, Simpson F, Sweetman EA, Davies AR. Early enteral nutrition, provided within 24 h of injury or intensive care unit admission, significantly reduces mortality in critically ill patients: a meta-analysis of randomised controlled trials. Intensive Care Med. 2009; 35:2018–27.

15. Khalid I, Doshi P, DiGiovine B. Early enteral nutrition and outcomes of critically ill patients treated with vasopressors and mechanical ventilation. Am J Crit Care. 2010; 19:261–8.

16. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012; 307:795–803.

Figure 1.
Protocol for enteral nutrition and residual volume check in new intensive care unit (ICU) evaluation & development of nutritional support protocol (NICE-NST) protocol. Enteral nutrition (EN) is supplied for 16 hours from 9 AM to 1 AM the next day. Gastric residual volume (GRV) check was done thrice daily at 9 AM, 5 PM, and 11 PM. If GRV is over 250 ml, EN feeding is held, and prokinetics are given thrice daily as scheduled by the nurse on duty. Two hours after withholding EN feeding, GRV recheck is performed, and the same protocol is repeated until 1 AM the next day.
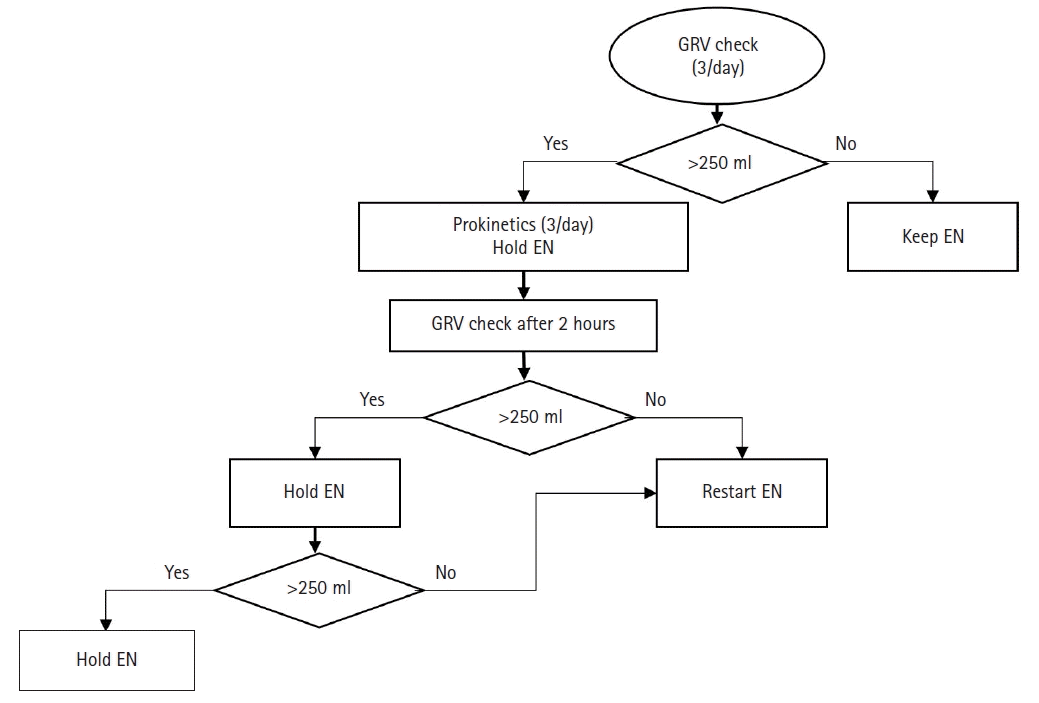
Figure 2.
Flowchart of the study participant selection. The control group was supplied nutrition without protocol, while the test group was supplied nutrition with protocol. ICU: intensive care unit; NST: nutrition support team; CRRT: continuous renal replacement therapy; HD: hemodialysis. Admission to NST time: the time duration from admission in ICU to NST consultation.
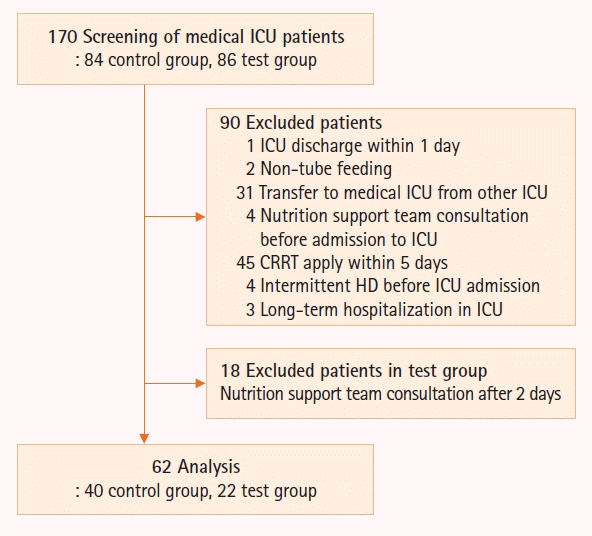
Figure 3.
Trends of supplying calorie and protein over time in control or test group. (A) Total calorie per weight supplied during intensive care unit (ICU) day 1 to 5. (B) Total protein per weight supplied during ICU day 1 to 5. (C) Enteral nutrition (EN) calorie per weight supplied during ICU day 1 to 5. (D) EN protein per weight supplied during ICU day 1 to 5. Differences between the two groups were tested with repeated-measures analysis of variance.
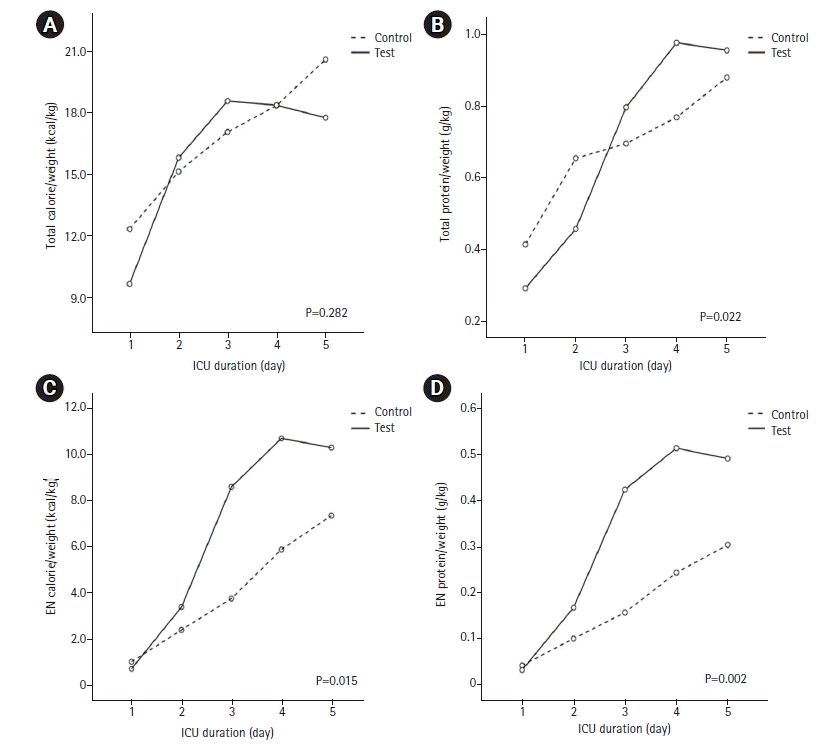
Table 1.
Protocol for categorizing patients in the NICE-NST protocol
| Variable |
Group 1 |
Group 2 |
|
|---|---|---|---|
| NUTRIC score <5 (or with abnormal liver function, or with abnormal renal function) | NUTRIC score ≥5 (or <70% of the daily nutritional requirement target after 7 days in ICU) | ||
| Route | EN | EN | Supplementary PN |
| Target | Target 100% of the required daily calories and 1.3 g/kg of protein | Target 100% of the required daily calories and 1.3 g/kg of protein | |
| Day 3 in ICU | Starting EN targeted 500 kcala | Starting EN targeted 500 kcala | Starting partial PN to meet nutritional targets |
Table 2.
Difference in the supply of calories and protein between control and test group
Table 3.
Clinical outcomes including mortalities and complications in the control and test group
| Variable | Control group (n=40) | Test group (n=22) | P-value |
|---|---|---|---|
| Admission to EN time (day)a | 3.7±0.4 | 2.4±0.5 | 0.010c |
| ICU duration (day)b | 19.2±7.9 | 15.4±4.5 | 0.848 |
| ICU mortality | 9 (22.5) | 4 (18.2) | 0.689 |
| Hospital mortality | 13 (32.5) | 8 (36.4) | 0.758 |
| Change of serum albumin level from 0 to 7 days (g/dl) | 0.24±0.08 | 0.25±0.06 | 0.893 |
| Hold of feeding due to excessive GRV or vomiting (n/day) | 0.47±0.28 | 0.10±0.10 | 0.256 |
| The ratio of hyperglycemia events (blood glucose >250 mg/dl) to total daily glucose check counts | 0.82±0.17 | 0.84±0.25 | 0.555 |
| The ratio of hypoglycemia events (blood glucose <70 mg/dl) to total daily glucose check counts | 0.06±0.03 | 0.01±0.01 | 0.114 |
Table 4.
Mortality according to admission to EN time within or more than 1.5 days
| Variable |
Admission to EN timea |
P-value | |
|---|---|---|---|
| >1.5 Days (n=63) | ≤1.5 Days (n=17) | ||
| ICU mortality | 18 (28.6) | 1 (5.9) | 0.051 |
| Hospital mortality | 27 (42.9) | 2 (11.8) | 0.018 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download