Abstract
Background/Aims
Acute-on-chronic liver failure (ACLF) is a widely recognized concept in which acute decompensation (AD) in patients with cirrhosis results in organ failure and high short-term mortality. On the other hand, few studies reflecting the various etiologies of cirrhosis are available. This study examined the clinical features of patients with hepatitis C virus (HCV)-related ACLF.
Methods
Between January 2005 and December 2018, 109 HCV-related cirrhosis patients hospitalized for AD (ascites, hepatic encephalopathy, gastrointestinal hemorrhage, and bacterial infection) were enrolled for ACLF defined by the European Association for the Study of the Liver (EASL).
Results
ACLF developed in 35 patients (32.1%) on admission. Eight, eight, and 19 patients had ACLF grades 1, 2, and 3, respectively. The 28-day and 90-day mortality rates were very low (2.7% and 5.4%, respectively) in patients without ACLF and very high (60.0% and 74.3%, respectively) in those with ACLF. In patients with HCV-related ACLF, compared to previous studies on hepatitis B virus-related ACLF and alcohol-related ACLF, the prevalence of liver failure was very low (17.1%), whereas that of kidney failure was very high (71.4%). Compared with all other prognostic scores, the Chronic liver failure Consortium Organ Failure score predicted the 90-day mortality most accurately, with an area under the receiver operator characteristic of 0.921.
Acute-on-chronic liver failure (ACLF) is an increasingly recognized syndrome in which acute decompensation (AD) in patients with chronic liver disease results in rapid organ failures associated with high short-term mortality.1-4 Patients with ACLF have 28-day and 90-day mortality rates of approximately 30% and in excess of 50%, respectively.5-7
On the other hand, there are no universal diagnostic criteria for ACLF. Recently, two definitions of ACLF by the Asian Pacific Association for the Study of the Liver (APASL) and the European Association for the Study of the Liver (EASL) were proposed and are currently widely accepted. APASL–ACLF was defined as an acute hepatic insult manifesting as jaundice (serum bilirubin ≥5 mg/dL) and coagulopathy (INR ≥1.5) complicated within four weeks by clinical ascites, or hepatic encephalopathy (HE), or both in patients with chronic liver disease.6 In contrast, the EASL–ACLF, from the CANONIC study, was defined as AD (HE, gastrointestinal (GI) hemorrhage, ascites, or bacterial infection) in cirrhosis patients, followed by the development of one or more organ failures.5
Another important problem beyond the ongoing controversies surrounding diverse ACLF definitions is that the data studied do not reflect the various causes of cirrhosis. The definition of APASL–ACLF was derived from a cohort of patients predominantly infected with the HBV. In contrast, in the EASL–ACLF cohort, approximately 60% of patients had alcoholic liver disease. Subsequently, the Chinese Group on the Study of Severe Hepatitis B (COSSH) developed a new definition for HBV-related ACLF.8 The Korean acute-on-chronic liver failure study cohort was proposed in cirrhosis patients from Korea, but this study had a population rate of alcoholic liver disease exceeding 60%.7,9 Lee et al.10 recently examined the ability of a chronic liver failure sequential organ failure assessment (CLIF–SOFA) to predict the short-term mortality in patients with alcohol-related ACLF.
On the other hand, no studies have included patients with HCV-related ACLF. Therefore, this study sought to identify the clinical features of patients of HCV-related ACLF in Korea, an HBV endemic area.
This retrospective cohort study included 1,743 patients with HCV infection who visited the Gyeongsang National University Changwon Hospital and Gyeongsang National University Hospital from January 2005 to December 2018. The exclusion criteria were as follows: 1) a follow-up period of less than 6 months (n=273); 2) presence of a hepatocellular carcinoma (n=143); 3) presence of an extrahepatic malignancy or severe extrahepatic disease (n=37); 4) HBV co-infection (n=53); 5) human immunodeficiency virus infection co-infection (n=5); and 6) acute HCV infection (n=10). Among the remaining 1,222 patients with chronic hepatitis C, 1,008 without cirrhosis and 214 with cirrhosis were initially analyzed for ACLF using the APASL criteria (total bilirubin ≥5 mg/dL and INR ≥1.5), applied to patients with chronic liver disease with or without cirrhosis. To apply the EASL–ACLF, after excluding 1,008 patients without cirrhosis and 105 patients without AD events as defined by the acute development of overt ascites, HE, GI hemorrhage, and bacterial infection, 109 patients with cirrhosis who developed AD were finally analyzed (Fig. 1). The study was approved by the Institutional Review Board (IRB) of Gyeongsang National University Changwon Hospital and Gyeongsang National University Hospital (IRB No. 2018-07-009; 2015-07-002). The need for informed consent was waived because of the retrospective design of this study, as determined by the IRB.
Data were collected from the medical charts, including patient demographics, clinical and laboratory data on admission, types of AD events and organ failures, potential precipitating factors of AD and ACLF, and development of ACLF. The potential precipitating factors included bacterial infections, GI hemorrhage, active alcoholism, large volume paracentesis without albumin, transjugular intrahepatic portosystemic shunting, major surgery, hepatitis (including reactivation of viral hepatitis and toxic liver injury), and alcoholic hepatitis. Active alcoholism was defined as >14 drinks per week in women and >21 drinks per week in men within the last 3 months.5 AD events were defined as acute onset of HE, ascites, GI hemorrhage, bacterial infection, or any combination of these. Organ failure was defined according to a modified CLIF Consortium Organ Failure score (CLIF-C OFs),11 which is a simplified modification of the CLIF–SOFA score and entails the following: liver failure, defined as a total bilirubin level of ≥12 mg/dL; kidney failure, defined as a serum creatinine level of ≥2.0 mg/dL, or requiring renal replacement therapy or both; cerebral failure, defined as grade III or IV HE based on the West Haven criteria; coagulation failure, defined as INR >2.5; circulation failure, defined as treatment with vasoconstrictors to maintain the arterial blood pressure or inotropes to improve cardiac output; and respiratory failure, defined as PaO2/FiO2 ≤200 or SpO2/FiO2 ≤214.
According to the EASL–ACLF criteria, the severity of ACLF was graded into ACLF grade 1 (ACLF-1), ACLF grade 2 (ACLF-2), or ACLF grade 3 (ACLF-3) according to the number of organ failures. ACLF-1 was defined by the presence of a single kidney failure or any other organ failure when in combination with either kidney dysfunction (serum creatinine ranging from 1.5 to 1.9 mg/dL) or grade I or II HE. ACLF-2 or 3 was defined by the presence of 2 or ≥3 organ failures, respectively. The ACLF and ACLF grades, as defined above, were examined by investigating any association of organ failure at admission.
The performance of CLIF-C OFs in evaluating prognosis was comparable to that of the CLIF–SOFA score.11 The short-term mortality in cirrhosis patients with AD was predicted by comparing the performance of CLLF-C OFs with that of Child– Turcotte–Pugh (CTP) scores, the model for end-stage liver disease (MELD) score, and the MELD-sodium (MELD-Na) score. In addition, the CLIF-C ACLF score (CLIF-C ACLFs) was used to predict short-term mortality in ACLF patients,11 and the CLIF-C AD score (CLIF-C ADs) was used in AD patients without ACLF.12
The following tests were performed to assess the association between patient characteristics and ACLF at admission: Fisher’s exact and Pearson’s chi-square tests to analyze the qualitative data and a Mann–Whitney U test to analyze the quantitative data. The survival rates for the development of 90-day survival were estimated by the Kaplan–Meier method and compared using the log-rank test. The accuracy of the CLIF-OFs, CTP score, MELD score, and MELD-Na score in predicting survival was assessed by the area under the receiver operating characteristic (AUROC) curve. A p-value <0.05 was considered significant for all analyses. All statistical operations were performed using PASW Statistics, version 18 (SPSS Inc., Chicago, IL, USA).
Patients with non-cirrhotic chronic hepatitis C did not exhibit ACLF as defined by APASL (total bilirubin ≥5 mg/dL and INR ≥1.5). Thus, the EASL–ACLF criteria were chosen to define ACLF in this study. Table 1 lists the baseline characteristics of 109 patients with HCV-related cirrhosis. Of cirrhosis patients with AD, ACLF developed in 35 patients (32.1%) upon admission. Eight patients (7.3%), eight (7.3%), and 19 (17.4%) had ACLF-1, 2, and 3, respectively. There was no significant difference in age, sex, HCV genotype, and sustained virologic response rate between patients with and without ACLF. Overt ascites were the most common type of AD, followed by bacterial infection, GI hemorrhage, and HE. GI hemorrhage, bacterial infection, and HE were more frequent in patients with ACLF than in patients without ACLF. A history of AD was reported in 35 patients (32.1%). Patients with ACLF more frequently had prior AD events.
Upon admission, patients with ACLF had higher median white blood cell, total bilirubin, creatinine, and INR levels but lower median albumin and sodium levels than those without ACLF (Table 2). The prognostic scores showed that patients with ACLF had higher CTP scores, MELD scores, MELD-Na scores, and CLIF-OFs than those without ACLF.
Patients with ACLF more frequently had bacterial infections, GI hemorrhage, and a composite of other precipitating events than those without ACLF. No precipitating event was observed in 28.4% of patients (Table 1). Pneumonia (21.6%) was the most common type of bacterial infection, followed by spontaneous bacterial peritonitis (18.9%), urinary tract infection (13.5%), unproved (13.5%), others (13.5%), and skin infection (10.8%) (Supplementary Table 1). The most common type of organ failure in patients with ACLF involved the kidney (71.4%), followed by the brain (54.3%), circulation (54.3%), the lungs (45.7%), coagulation (34.3%), and the liver (17.1%). The causes of AKI were prerenal in six patients (24.0%), hepato-renal syndrome in 10 patients (40.0%), infection in eight patients (32.0%), and unknown in one patient (4.0%).
Kaplan–Meier curves of the probability of survival showed that patients with ACLF had poorer outcomes than those with AD (Fig. 2A). The mortality at 28 days, 90 days, and 1 year for patients without ACLF was 2.7%, 5.4%, and 9.5%, respectively while the mortality at 28 days, 90 days, and 1 year for patients with ACLF was 60.0%, 74.3%, and 80.0%, respectively (Fig. 2B). The mortality at 28 days and 90 days was 2.7% and 5.4% for patients without ACLF, 0% and 37.5% for those with ACLF-1, 75.0% and 87.5% for those with ACLF-2, and 78.9% and 89.5% for those with ACLF-3, respectively (Supplementary Fig. 1). In the survival curve according to prior AD, there was a significant difference in the survival rates of patients with or without ACLF, but there was no significant difference in the survival rates of patients according to prior AD (Supplementary Fig. 2). Multiple organ failure without septic shock or hypovolemic shock was the most common cause of death at 90 days (53.3%), followed by septic shock (20.0%), and hypovolemic shock (16.7%) (Supplementary Table 2).
The median CLIF-C ADs in patients without ACLF (n=74) and CLIF-C ACLFs in patients with ACLF (n=35) were 48.0 and 54.0, respectively. A strong stepwise association was observed between CLIF-C OFs and the ACLF grades in cirrhosis patients with AD (SupplementaryFig. 3). The median CLIF-C OFs were 6.0, 7.5, 10.0, and 13.0 in patients without ACLF, ACLF-1, ACLF-2, and ACLF-3, respectively. All prognostic scores, including CTP score, MELD score, MELD-Na score, and CLIF-C OFs, were significantly higher in patients with ACLF than those without ACLF (Table 2). In all patients (n=109), the median CLIF-C OFs were significantly higher in patients who died within 90 days than in those who survived (12.0 vs. 6.0, p<0.001) (Supplementary Fig. 4A). In patients without ACLF, there was no significant difference in the median CLIF-ADs between patients who died and survived (49.5 vs. 48.0, p=0.881) (Supplementary Fig. 4B). In patients with ACLF, there was no significant difference in median CLIF– ACLFs between patients who did and did not die (54.0 vs. 47.0, p=0.342) (Supplementary Fig. 4C). The baseline data, Fig. 3. Receiver operating characteristic curves of the CLIF-C OF score and three prognostic scoring systems in predicting the 90-day mortality in HCV-related cirrhosis with acute decompensation (n=109). CLIF-C OF, chronic liver failure Consortium Organ Failure score; MELD, model for end-stage liver disease; CTP, Child–Turcotte –Pugh; HCV, hepatitis C virus. defined as pre-specified information at 3 months (up to 6 months) prior to enrollment, were available for 39 patients (Supplementary Table 3). Prognostic scores at baseline revealed that patients with ACLF had higher CTP scores, MELD scores, and MELD-Na scores than those without ACLF. Among all prognostic parameters in all patients, CLIF-C OFs revealed the highest AUROC (0.921; 95% CI, 0.855-0.986) for predicting 90-day mortality (Fig. 3).
ACLF, as defined by APASL, developed in 10 patients (9.2%) at admission in 109 patients with AD and cirrhosis. The mortality at 28 days, 90 days, and 1 year for patients without APASL–ACLF was 17.2%, 21.2%, and 26.3%, respectively, while the mortality at 28 days, 90 days, and 1 year for patients with APASL–ACLF was 60.0%, 90.0%, and 90.0%, respectively (Supplementary Fig. 5).
In this study of 109 patients with HCV-related cirrhosis who were hospitalized for AD (ascites, HE, GI hemorrhage, and bacterial infection), 28-day and 90-day mortalities were higher in patients with ACLF at admission than those without ACLF (60.0% and 74.3% vs. 2.7% and 5.4%, respectively). In addition, the CLIF-C OFs were the most accurate in predicting the 90-day mortality for HCV-related cirrhosis patients with AD compared to the CTP score, MELD score, and MELD-Na score.
Among the various definitions of ACLF, no studies were conducted in a cohort consisting only of patients with HCV-related chronic liver disease. In the chronic hepatitis C cohort of 1,222 patients, no patient met the definition of APASL–ACLF (total bilirubin ≥5 mg/dL and INR ≥1.5) in patients without cirrhosis. There are very few episodes of acute flare-ups in chronic hepatitis C patients, even in immunocompromised patients.13 Therefore, non-cirrhotic HCV–ACLF rarely occurs in chronic hepatitis C without cirrhosis, unlike in non-cirrhotic HBV–ACLF.8,14,15 This suggests that the APASL–ACLF or COSSH criteria cannot be applied to patients with non-cirrhotic chronic hepatitis C. Among the 109 HCV-related cirrhosis patients, eight had EASL–ACLF and APASL–ACLF, 27 had EASL–ACLF alone, and two had APASL–ACLF alone. Therefore, the EASL– ACLF criteria detected more ACLF patients even in the setting of chronic hepatitis with cirrhosis. In a previous study using data from the Veterans Health Administration, the incidence of ACLF for patients with hepatitis C was higher in the EASL–ACLF criteria than in APASL–ACLF.16 In this study, patients with EASL–ACLF upon admission had a significantly higher 90-day mortality rate than patients without EASL–ACLF. In particular, patients with ACLF-2 and ACLF-3 upon admission had extremely high 90-day mortality (87.5% and 89.5%, respectively), while patients with no ACLF had a very low 90-day mortality (5.4%). These suggest that EASL–ACLF is a very useful tool for predicting the prognosis in HCV-related cirrhosis patients hospitalized for acute deterioration. To the best of the authors’ knowledge, this study is the first on HCV-related ACLF that does not contain ACLF with other etiologies.
HCV-related ACLF showed distinctive characteristics that distinguished ACLF from other causes. The 90-day mortality for patients with ACLF was highest in the present study, composed of HCV-related ACLF (74.3%), compared with the COSSH study (HBV-related ACLF, 69.7%),8 the Korean study (alcohol-related ACLF, 67.2%),10 and the CANONIC study, composed of various etiologies (51.2%).5 Compared to the prevalence of organ failure, liver failure in HCV-related ACLF was very low (17.1%) compared to HBV-related ACLF (93.7%) and the CANONIC study (43.6%).5,8 On the other hand, the prevalence of kidney failure in HCV-related ACLF was very high (71.4%) compared to that of HBV-related ACLF (14.0%) and the CANONIC study (55.8%). Therefore, ACLF in HCV-related cirrhosis may be associated with kidney failure rather than liver failure, which is believed to be associated with high short-term mortality. HCV infection is a systemic disease characterized by metabolic diseases. A previous study17 reported that the incidence of AKI in patients with chronic hepatitis C was 4.35 per 100 person-years (compensated cirrhosis, 5.86 per 100 person-years; and decompensated cirrhosis, 17.28 per 100 person-years). The prevalence of AKI in patients with chronic hepatitis C was 22.8%. AKI events are common in the natural history of chronic hepatitis C, and AKI has a significant effect on mortality. These suggest that the mechanism for HCV-related ACLF probably reflects an extra-hepatic insult, such as chronic kidney impairment, worsening hepatic function, ascites, low arterial pressure, shock, low serum sodium levels, infection, and use of antiviral agents, while the mechanism for HBV-related ACLF probably reflects a hepatic insult, such as HBV flare up. On the other hand, a direct comparison of the characteristics between HCV-related ACLF and HBV-or alcohol-related ACLF is impossible due to the lack of data on our population. In a recent large-scale retrospective cohort study in the United States, patients with hepatitis C had the lowest ACLF incidence rate but had the highest short-term mortality compared with patients with HBV-related ACLF and alcohol-related ACLF.16
CLIF-C OFs displayed the best prognostic ability for cirrhosis patients with AD (AUROC, 0.921; 95% CI, 0.855-0.986) compared to the CTP score, MELD score, and MELD-Na score. CTP, MELD, and MELD-Na scores are based only on liver failure (bilirubin), kidney failure (creatinine), coagulation failure (INR), and cerebral failure (HE). In contrast, CLIF-C OFs also reflect coagulation and respiratory failure to predict the prognosis more effectively. The CLIF–SOFA score is a widely used tool for predicting short-term mortality in ACLF and AD patients and is superior to the MELD score in predicting the prognosis.10,14,18,19 This study showed that short-term mortality could be effectively predicted using CLIF-C OFs, a simplified modification of the CLIF–SOFA score. In addition, CTP, MELD, and MELD-Na score 3 months prior to enrollment were higher in patients with ACLF than in those without ACLF. This finding suggests that patients with ACLF were already more critically ill than patients without ACLF at baseline before reaching the ACLF status.
This study had some limitations. First, this was a retrospective study with relatively small sample size. Owing to an insufficient number of patients, there were no significant differences in prognostic scores between patients who survived and those who died. This study could not accurately access HE grades 1 and 2 for measuring the CLIF-C OFs through a retrospective chart review. Second, patients lost to follow-up within 6 months after transferring to other hospitals for liver transplantation were excluded because the institute cannot perform liver transplantation. Third, most HCV-infected patients in this study did not receive antiviral therapy because they were enrolled before the direct-acting agent era or had severely decompensated cirrhosis. Despite these limitations, this study is the first study to identify the clinical features of patients with HCV-related ACLF, especially in Korea, an HBV endemic area.
In conclusion, applying the EASL–ACLF definition to patients with HCV-related cirrhosis can be useful for predicting the short-term mortality, consistent with previous studies conducted on the other etiologies. Furthermore, HCV-related ACLF has unique clinical features distinct from HBV-related or alcohol-related ACLF.
REFERENCES
1. Arroyo V, Moreau R, Jalan R. 2020; Acute-on-chronic liver failure. N Engl J Med. 382:2137–2145. DOI: 10.1056/NEJMra1914900. PMID: 32459924.
2. Gustot T, Moreau R. 2018; Acute-on-chronic liver failure vs. traditional acute decompensation of cirrhosis. J Hepatol. 69:1384–1393. DOI: 10.1016/j.jhep.2018.08.024. PMID: 30195459.
3. Hernaez R, Solà E, Moreau R, Ginès P. 2017; Acute-on-chronic liver failure: an update. Gut. 66:541–553. DOI: 10.1136/gutjnl-2016-312670. PMID: 28053053. PMCID: PMC5534763.
4. Bernal W, Jalan R, Quaglia A, Simpson K, Wendon J, Burroughs A. 2015; Acute-on-chronic liver failure. Lancet. 386:1576–1587. DOI: 10.1016/S0140-6736(15)00309-8.
5. Moreau R, Jalan R, Gines P, et al. 2013; Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 144:1426–1437. e14379. DOI: 10.1053/j.gastro.2013.02.042. PMID: 23474284.
6. Sarin SK, Choudhury A, Sharma MK, et al. 2019; Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 13:353–390. DOI: 10.1007/s12072-019-09946-3. PMID: 31172417. PMCID: PMC6728300.
7. Kim TY, Song DS, Kim HY, et al. 2016; Characteristics and discrepancies in acute-on-chronic liver failure: need for a unified definition. PLoS One. 11:e0146745. DOI: 10.1371/journal.pone.0146745. PMID: 26789409. PMCID: PMC4720429.
8. Wu T, Li J, Shao L, et al. 2018; Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 67:2181–2191. DOI: 10.1136/gutjnl-2017-314641. PMID: 28928275.
9. Yoon EL, Kim TY, Lee CH, et al. 2019; Long-term prognosis of acute-on-chronic liver failure survivors. J Clin Gastroenterol. 53:134–141. DOI: 10.1097/MCG.0000000000000987. PMID: 29369242. PMCID: PMC6358187.
10. Lee M, Lee JH, Oh S, et al. 2015; CLIF-SOFA scoring system accurately predicts short-term mortality in acutely decompensated patients with alcoholic cirrhosis: a retrospective analysis. Liver Int. 35:46–57. DOI: 10.1111/liv.12683. PMID: 25203221.
11. Jalan R, Saliba F, Pavesi M, et al. 2014; Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 61:1038–1047. DOI: 10.1016/j.jhep.2014.06.012. PMID: 24950482.
12. Jalan R, Pavesi M, Saliba F, et al. 2015; The CLIF consortium acute decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 62:831–840. DOI: 10.1016/j.jhep.2014.11.012. PMID: 25463539.
13. Massard J, Ratziu V, Thabut D, et al. 2006; Natural history and predictors of disease severity in chronic hepatitis C. J Hepatol. 44(Suppl 1):S19–S24. DOI: 10.1016/j.jhep.2005.11.009. PMID: 16356583.
14. Li H, Chen LY, Zhang NN, et al. 2016; Characteristics, diagnosis and prognosis of acute-on-chronic liver failure in cirrhosis associated to hepatitis B. Sci Rep. 6:25487. DOI: 10.1038/srep25487. PMID: 27146801. PMCID: PMC4857102.
15. Zhao RH, Shi Y, Zhao H, Wu W, Sheng JF. 2018; Acute-on-chronic liver failure in chronic hepatitis B: an update. Expert Rev Gastroenterol Hepatol. 12:341–350. DOI: 10.1080/17474124.2018.1426459. PMID: 29334786.
16. Mahmud N, Kaplan DE, Taddei TH, Goldberg DS. 2019; Incidence and mortality of acute-on-chronic liver failure using two definitions in patients with compensated cirrhosis. Hepatology. 69:2150–2163. DOI: 10.1002/hep.30494. PMID: 30615211. PMCID: PMC6461492.
17. Jeon H, Kim JH, Lee SS, et al. 2021; Impact of acute kidney injury on survival in patients with chronic hepatitis C: a retrospective cohort study. BMC Infect Dis. 21:301. DOI: 10.1186/s12879-021-05991-2. PMID: 33765952. PMCID: PMC7993493.
18. Engelmann C, Thomsen KL, Zakeri N, et al. 2018; Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit Care. 22:254. DOI: 10.1186/s13054-018-2156-0. PMID: 30305132. PMCID: PMC6180662.
19. Shin J, Yu JH, Jin YJ, et al. 2020; Acute-on-chronic liver failure as a major predictive factor for mortality in patients with variceal bleeding. Clin Mol Hepatol. 26:540–553. DOI: 10.3350/cmh.2020.0034. PMID: 32937688. PMCID: PMC7641565.
Fig. 1
Flow sheet. HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HIV, human immunodeficiency virus; HCV, hepatitis C virus; ACLF, acute-on-chronic liver failure; APASL, Asian Pacific Association for the Study of the Liver.
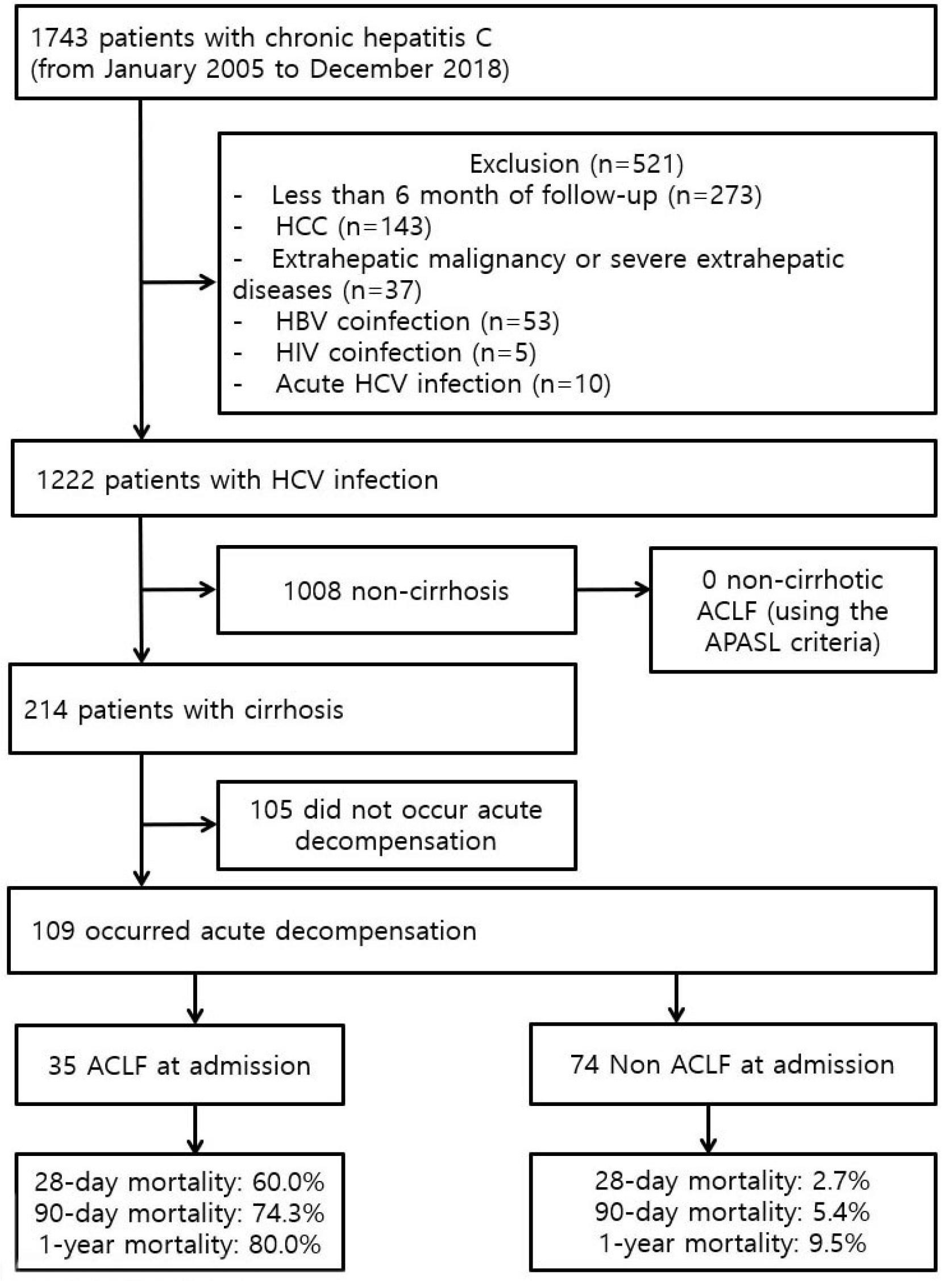
Fig. 2
Prognosis according to ACLF. (A) Kaplan–Meier curves of the probability of survival within 90 days. (B) Mortality at 28 days, 90 days, and 1 year of patients without or with ACLF. ACLF, acute-on-chronic liver failure.
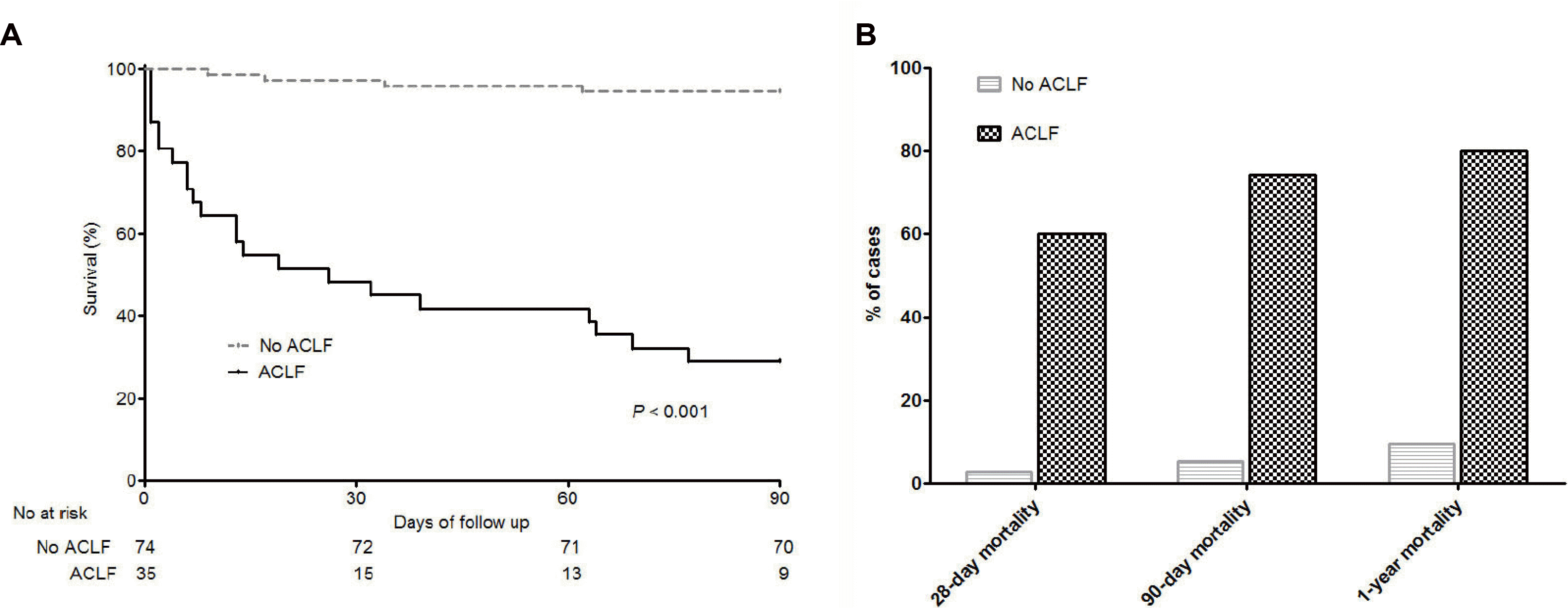
Fig. 3
Receiver operating characteristic curves of the CLIF-C OF score and three prognostic scoring systems in predicting the 90-day mortality in HCV-related cirrhosis with acute decompensation (n=109). CLIF-C OF, chronic liver failure Consortium Organ Failure score; MELD, model for end-stage liver disease; CTP, Child–Turcotte –Pugh; HCV, hepatitis C virus.
Table 1
Baseline Characteristics of Patients with HCV-related Cirrhosis at Admission according to ACLF
| Characteristic | Overall (n=109) | No ACLF (n=74) | ACLF (n=35) | p-valuea |
|---|---|---|---|---|
| Age (years) | 62.0 (53.0-71.5) | 63.0 (53.0-72.3) | 61.0 (51.0-70.0) | 0.638 |
| Male sex | 65 (59.6) | 43 (58.1) | 22 (62.9) | 0.680 |
| HCV genotype | 0.594 | |||
| 1 | 50 (45.9) | 32 (43.2) | 18 (51.4) | |
| 2 | 45 (41.3) | 33 (44.6) | 12 (34.3) | |
| 3 | 14 (12.8) | 9 (12.2) | 5 (14.3) | |
| SVR at enrollment | 12 (11.0) | 7 (9.5) | 5 (14.3) | 0.517 |
| Causes of hospitalization | ||||
| Ascites | 50 (45.9) | 36 (48.6) | 14 (40.0) | 0.419 |
| HE | 24 (22.0) | 7 (9.5) | 17 (48.6) | <0.001 |
| GI hemorrhage | 36 (33.0) | 30 (40.5) | 6 (17.1) | 0.017 |
| Bacterial infection | 37 (33.9) | 17 (23.0) | 20 (57.1) | 0.001 |
| Precipitating events | ||||
| Bacterial infection | 37 (33.9) | 17 (23.0) | 20 (57.1) | 0.001 |
| GI hemorrhage | 36 (33.0) | 30 (40.5) | 6 (17.1) | 0.017 |
| Active alcoholism | 11 (10.1) | 7 (9.5) | 4 (11.4) | 0.743 |
| Other precipitating events | 5 (4.6) | 1 (1.4) | 4 (11.4) | 0.036 |
| No precipitating event | 31 (28.4) | 24 (32.4) | 7 (20.0) | 0.256 |
| More than one precipitating event | 9 (8.3) | 4 (5.4) | 5 (14.3) | 0.143 |
| Organ failure | ||||
| Liver | 7 (6.4) | 1 (1.4) | 6 (17.1) | 0.004 |
| Kidney | 25 (22.9) | 0 (0.0) | 25 (71.4) | <0.001 |
| Cerebral | 26 (23.9) | 7 (9.5) | 19 (54.3) | <0.001 |
| Coagulation | 12 (11.0) | 0 (0.0) | 12 (34.3) | <0.001 |
| Circulation | 19 (17.4) | 0 (0.0) | 19 (54.3) | <0.001 |
| Respiration | 17 (15.6) | 1 (1.4) | 16 (45.7) | <0.001 |
| Kidney dysfunction | 8 (7.3) | 4 (5.4) | 4 (11.4) | 0.267 |
| Time from first previous AD | <0.001 | |||
| No previous AD | 74 (67.9) | 59 (79.7) | 15 (42.9) | |
| Less than 12 months | 14 (12.8) | 7 (9.5) | 7 (20.0) | |
| More than 12 months | 21 (19.3) | 8 (10.8) | 13 (37.1) |
Table 2
Prognostic Scores and Laboratory Data at Admission
| Characteristic | Overall (n=109) | No ACLF (n=74) | ACLF (n=35) | p-valuea |
|---|---|---|---|---|
| Prognostic scores | ||||
| CTP | 9.0 (7.0-11.0) | 8.0 (7.0-10.0) | 10.0 (8.0-12.0) | <0.001 |
| MELD | 14.0 (9.5-21.5) | 11.0 (9.0-15.3) | 26.0 (20.0-31.0) | <0.001 |
| MELD-Na | 18.0 (12.0-25.0) | 14.5 (11.0-18.3) | 28.0 (22.0-33.0) | <0.001 |
| CLIF-C OFs | 6.0 (6.0-8.5) | 6.0 (6.0-6.0) | 9.0 (12.0-14.0) | <0.001 |
| CLIF-C ADs | 48.0 (44.0-54.0) | |||
| CLIF-C ACLFs | 54.0 (46.0-61.0) | |||
| Laboratory data | ||||
| WBC (10×109/L) | 6.3 (4.3-9.8) | 5.7 (4.2-8.0) | 9.4 (4.9-12.5) | 0.005 |
| Hemoglobin (g/dL) | 10.7 (8.8-12.3) | 11.2 (8.8-12.7) | 10.1 (8.6-11.8) | 0.150 |
| Platelet (×109/L) | 107.0 (67.0-136.5) | 109.0 (70.0-139.5) | 100.0 (54.0-127.0) | 0.270 |
| Bilirubin (mg/dL) | 1.6 (0.8-3.1) | 1.3 (0.6-2.9) | 2.4 (1.3-5.0) | 0.001 |
| AST (U/L) | 59.0 (38.0-96.0) | 57.5 (37.8-84.5) | 60.0 (38.0-161.0) | 0.345 |
| ALT (U/L) | 35.0 (20.0-62.0) | 35.0 (20.0-53.5) | 28.0 (38.0-161.0) | 0.820 |
| Albumin (g/dL) | 2.7 (2.5-3.1) | 2.9 (2.6-3.1) | 2.5 (2.1-2.8) | <0.001 |
| Creatinine (mg/dL) | 0.90 (0.69-1.64) | 0.76 (0.62-0.90) | 2.33 (1.69-2.96) | <0.001 |
| Sodium (mmol/L) | 136.0 (132.0-139.4) | 136.3 (133.4-139.3) | 133.2 (127.5-140.1) | 0.045 |
| PT-INR | 1.42 (1.21-1.73) | 1.31 (1.17-1.56) | 1.81 (1.39-2.61) | <0.001 |
Values are presented as the median (interquartile range) for continuous data and percentages for categorical data.
ACLF, acute on chronic liver failure; CTP, Child-Turcotte-Pugh; MELD, model for end-stage liver disease; MELD-Na, model for end-stage liver disease-sodium; CLIF-C OFs, Chronic Liver Failure-Consortium Organ Failure Score; CLIP-C ADs, CLIF Consortium Acute Decompensation score; CLIP-C ACLFs, CLIF-Consortium scores for ACLF; WBC, white blood cell; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; PT-INR, prothrombin time-international normalized ratio.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download