1. Humphris JL, Jones DB. Subepithelial mass lesions in the upper gastrointestinal tract. J Gastroenterol Hepatol. 2008; 23:556–566.
2. Hawes R, Fockens P, Varadarajulu S. Endosonography. 4th ed. Philadelphia: Elsevier;2018.
3. Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005; 37:635–645.
4. Buscaglia JM, Nagula S, Jayaraman V, et al. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest Endosc. 2012; 75:1147–1152.
5. Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007; 13:2077–2082.
6. Attila T, Aydın Ö. Lesion size determines diagnostic yield of EUS-FNA with onsite cytopathologic evaluation for upper gastrointestinal subepithelial lesions. Turk J Gastroenterol. 2018; 29:436–441.
7. van Riet PA, Erler NS, Bruno MJ, et al. Comparison of fine-needle aspiration and fine-needle biopsy devices for endoscopic ultrasound-guided sampling of solid lesions: a systemic review and meta-analysis. Endoscopy. 2021; 53:411–423.
8. Facciorusso A, Sunny SP, Del Prete V, et al. Comparison between fine-needle biopsy and fine-needle aspiration for EUS-guided sampling of subepithelial lesions: a meta-analysis. Gastrointest Endosc. 2020; 91:14–22.
9. Zhang XC, Li QL, Yu YF, et al. Diagnostic efficacy of endoscopic ultrasound-guided needle sampling for upper gastrointestinal subepithelial lesions: a meta-analysis. Surg Endosc. 2016; 30:2431–2441.
10. Yokohata N, Tamegai Y, Tokuhara M, et al. 3 case of gastric SMT (submucal tumor) which was diagnoced before operation: open biopsy with ESD (endoscopic submucal dissection) for interstind SMT. Prog Dig Endosc. 2007; 70:82–83.
11. Ihara E, Matsuzaka H, Honda K, et al. Mucosal-incision assisted biopsy for suspected gastric gastrointestinal stromal tumors. World J Gastrointest Endosc. 2013; 5:191–196.
12. Dhaliwal A, Kolli S, Dhindsa BS, et al. Clinical efficacy and safety of mucosal incision-assisted biopsy for the diagnosis of upper gastrointestinal subepithelial tumors: a systematic review and meta-analysis. Ann Gastroenterol. 2020; 33:155–161.
13. Dhaliwal A, Kolli S, Dhindsa BS, et al. Diagnostic yield of deep biopsy via endoscopic submucosal dissection for the diagnosis of upper gastrointestinal subepithelial tumors: a systematic review and meta-analysis. Ann Gastroenterol. 2020; 33:30–37.
14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71.
15. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.
16. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016; 355:i4919.
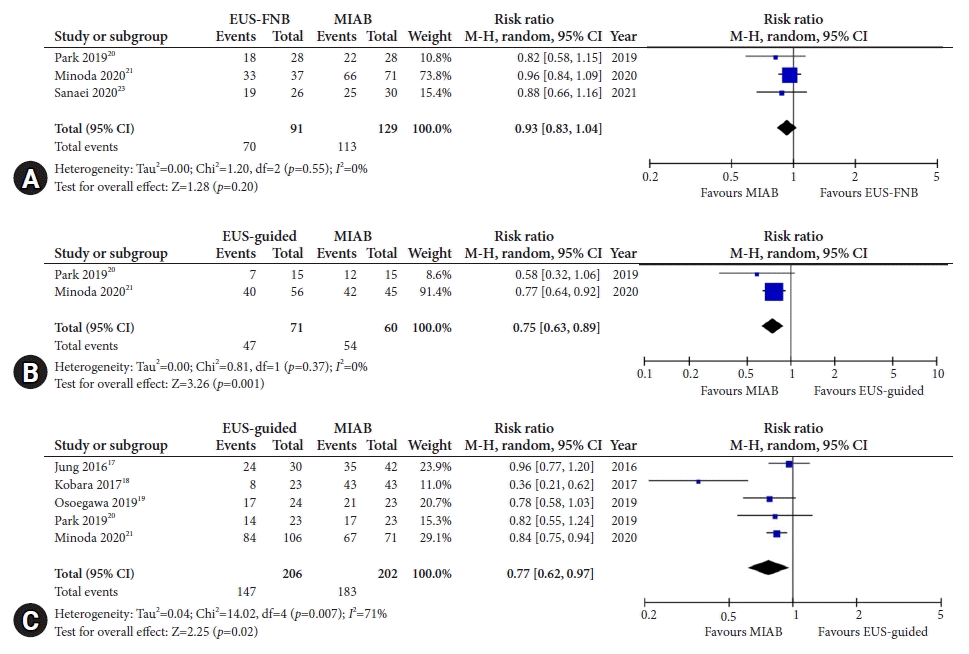
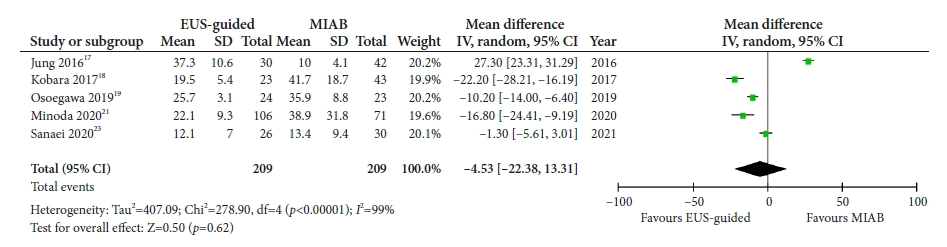
17. Jung YS, Lee H, Kim K, et al. Using forceps biopsy after small submucosal dissection in the diagnosis of gastric subepithelial tumors. J Korean Med Sci. 2016; 31:1768–1774.
18. Kobara H, Mori H, Nishimoto N, et al. Comparison of submucosal tunneling biopsy versus EUS-guided FNA for gastric subepithelial lesions: a prospective study with crossover design. Endosc Int Open. 2017; 5:E695–E705.
19. Osoegawa T, Minoda Y, Ihara E, et al. Mucosal incision-assisted biopsy versus endoscopic ultrasound-guided fine-needle aspiration with a rapid on-site evaluation for gastric subepithelial lesions: a randomized cross-over study. Dig Endosc. 2019; 31:413–421.
20. Park J, Park JC, Jo JH, et al. Prospective comparative study of endoscopic ultrasonography-guided fine-needle biopsy and unroofing biopsy. Dig Liver Dis. 2019; 51:831–836.
21. Minoda Y, Chinen T, Osoegawa T, et al. Superiority of mucosal incision-assisted biopsy over ultrasound-guided fine needle aspiration biopsy in diagnosing small gastric subepithelial lesions: a propensity score matching analysis. BMC Gastroenterol. 2020; 20:19.
22. Zoundjiekpon V, Falt P, Fojtik P, et al. Endosonography-guided fine-needle aspiration versus “Key-Hole Biopsy” in the diagnostics of upper gastrointestinal subepithelial tumors: a prospective randomized interventional study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020; 164:63–70.
23. Sanaei O, Fernández-Esparrach G, De La Serna-Higuera C, et al. EUS-guided 22-gauge fine needle biopsy versus single-incision with needle knife for the diagnosis of upper gastrointestinal subepithelial lesions: a randomized controlled trial. Endosc Int Open. 2020; 8:E266–E273.
24. Deprez PH, Moons LM, OʼToole D, et al. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022; 54:412–429.
25. Casali PG, Blay JY, Abecassis N, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022; 33:20–33.
26. Nishida T, Hirota S, Yanagisawa A, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008; 13:416–430.
27. Kim HH. Endoscopic treatment for gastrointestinal stromal tumor: advantages and hurdles. World J Gastrointest Endosc. 2015; 7:192–205.
28. Facciorusso A, Crinò SF, Ramai D, et al. Comparison between endoscopic ultrasound-guided fine-needle biopsy and bite-on-bite jumbo biopsy for sampling of subepithelial lesions. Dig Liver Dis. 2022; 54:676–683.
29. Larghi A, Fuccio L, Chiarello G, et al. Fine-needle tissue acquisition from subepithelial lesions using a forward-viewing linear echoendoscope. Endoscopy. 2014; 46:39–45.
30. Matsuzaki I, Miyahara R, Hirooka Y, et al. Forward-viewing versus oblique-viewing echoendoscopes in the diagnosis of upper GI subepithelial lesions with EUS-guided FNA: a prospective, randomized, crossover study. Gastrointest Endosc. 2015; 82:287–295.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download