Abstract
Background
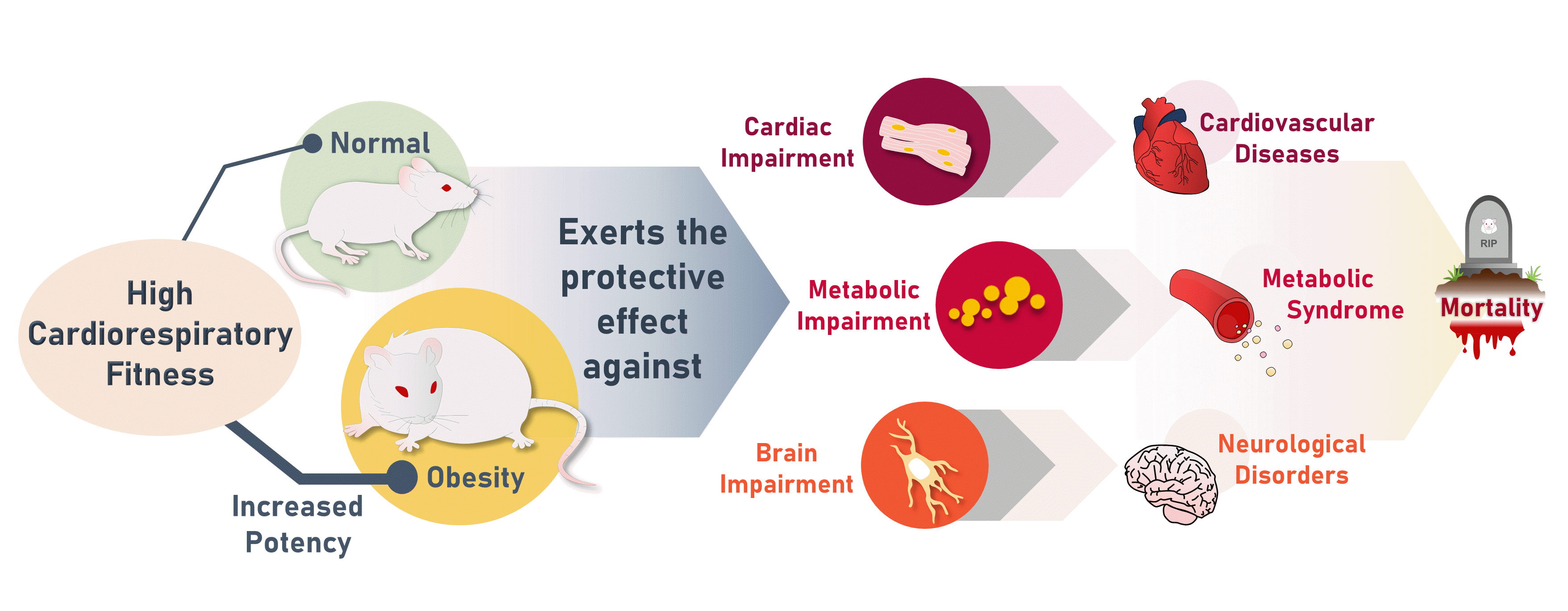
High cardiorespiratory fitness (CRF) protects against age-related diseases. However, the mechanisms mediating the protective effect of high intrinsic CRF against metabolic, cardiac, and brain impairments in non-obese versus obese conditions remain incompletely understood. We aimed to identify the mechanisms through which high intrinsic CRF protects against metabolic, cardiac, and brain impairments in non-obese versus obese untrained rats.
Methods
Seven-week-old male Wistar rats were divided into two groups (n=8 per group) to receive either a normal diet or a high-fat diet (HFD). At weeks 12 and 28, CRF, carbohydrate and fatty acid oxidation, cardiac function, and metabolic parameters were evaluated. At week 28, behavior tests were performed. At the end of week 28, rats were euthanized to collect heart and brain samples for molecular studies.
Results
The obese rats exhibited higher values for aging-related parameters than the non-obese rats, indicating that they experienced obesity-induced premature aging. High baseline CRF levels were positively correlated with several favorable metabolic, cardiac, and brain parameters at follow-up. Specifically, the protective effects of high CRF against metabolic, cardiac, and brain impairments were mediated by the modulation of body weight and composition, the lipid profile, substrate oxidation, mitochondrial function, insulin signaling, autophagy, apoptosis, inflammation, oxidative stress, cardiac function, neurogenesis, blood-brain barrier, synaptic function, accumulation of Alzheimer’s disease-related proteins, and cognition. Interestingly, this effect was more obvious in HFD-fed rats.
Cardiorespiratory fitness (CRF) represents the ability of the cardiovascular, respiratory, and muscular systems to supply oxygen during exercise [1]. It has been demonstrated that CRF is significantly genetically determined, with a heritability of 72% [2]. Previous longitudinal studies have shown that people with higher CRF levels had lower risks of metabolic syndrome, cardiovascular diseases, dementia, and all-cause mortality [3-5], suggesting that high CRF potentially protects against metabolic, cardiac, and brain impairments.
Previous studies investigating the mechanisms responsible for high CRF-induced delayed metabolic impairment were established using rats selectively bred for high and low intrinsic running capacity (HCR and LCR) [6]. It was observed that HCR rats had lower adiposity, higher insulin sensitivity, and a more favorable lipid profile [6-9]. Moreover, HCR rats had greater energy expenditure and heat production in skeletal muscle during exercise [10]. Metabolomic and proteomic analyses also revealed that skeletal muscle from HCR rats exhibited a higher capacity for utilization of fatty acids and branched-chain amino acids [11]. These findings may all be mechanistically linked to the protective effect of high CRF against metabolic syndrome. However, the effect of CRF has never been compared between non-obese and obese conditions, although obesity is known to accelerate aging [12]. The protective role of CRF in non-selectively bred animals remains unknown. The hearts of HCR rats have been shown to be characterized by higher respiration, greater antioxidative capacity, a lower degree of remodeling, and more favorable systolic and diastolic function [13,14]. Regarding the brain, HCR rats displayed a higher neuronal number, a greater hippocampal volume, a higher mitochondrial DNA number, higher respiration, lower expression of Alzheimer’s disease (AD)-related proteins, and better cognition [15,16]. The above findings imply that some molecular mechanisms potentially underlying the effect of high CRF on protection against cardiac and brain impairments have not yet been determined. As is the case with metabolic impairment, this effect of CRF has been neither compared between non-obese conditions and obesity-induced premature aging, nor evaluated in non-selectively bred animals.
Therefore, the aim of this study was to investigate the mechanisms mediating the effect of high intrinsic CRF on protection against metabolic, cardiac, and neuronal dysfunction in non-obese versus obese untrained Wistar rats. We hypothesized that a high intrinsic CRF level would protect against metabolic, cardiac, and neuronal impairment by modulating several mechanisms, and importantly, that these effects of high intrinsic CRF would also be observed in obesity-induced premature aging.
The experiments were performed at the laboratory animal center of Chiang Mai University, Thailand following a protocol approved by the Animal Care and Use Committee of Chiang Mai University, Chiang Mai, Thailand (2563/RT-0006). Male Wistar rats (n=16) were purchased from Nomura Siam International Co. Ltd. (Bangkok, Thailand).
The study protocol is illustrated in Supplemental Fig. S1. Male Wistar rats at the age of 7 weeks (n=16) were randomly assigned into two equal groups (n=8 per group). The first group received an ad libitum normal diet (ND) containing 19.7% energy from fat. The second group received an ad libitum high-fat diet (HFD) containing 59.28% energy from fat. The HFD was made by the laboratory animal center of Chiang Mai University, Chiang Mai, Thailand. At weeks 12 and 28, CRF and whole-body substrate oxidation during exercise were evaluated. Cardiac function was also investigated at weeks 12 and 28 using echocardiography and heart rate variability (HRV). Additionally, blood was collected by cutting the tip of the tail after 5 hours of fasting at weeks 12 and 28 to measure metabolic parameters, including the insulin sensitivity profile and lipid profile. At week 28, behavior tests including an open-field test (OFT) and modified novel object location (NOL) and novel object recognition (NOR) tests for cognitive function were performed. At the end of week 28, all rats were euthanized to enable the collection of heart and brain tissues for molecular studies. Regular insulin (10 U/kg; Actrapid HM, Novo Nordisk, Copenhagen, Denmark) was intraperitoneally injected into the rats 30 minutes before decapitation.
The details regarding the general methods are described in the Supplemental Methods. These methods include CRF level measurements, whole-body substrate oxidation during vigorous exercise, plasma insulin sensitivity and lipid profiles, echocardiography and HRV, behavior and cognitive tests (OFT, NOL, and NOR), measurement of malondialdehyde concentrations in cardiac and brain tissues, protein expression analyses, cardiac mitochondrial function and mitochondrial respiration assay, hippocampal reactive oxygen species levels, ex vivo brain incubation with regular insulin solution, and an apoptotic cell death assay.
For comparisons between the different time points (baseline at week 12 vs. follow-up at week 28) within the same group, data were analyzed using the paired Student’s t test. For comparisons between ND-versus HFD-fed rats, and between subgroups of rats with higher baseline CRF levels (ranked first to fourth in baseline running distance; n=4) versus those with lower baseline CRF levels (ranked fifth to eighth in baseline running distance; n=4) within each diet group, the unpaired two-tailed Student’s t test was performed. Correlations within each group of rats were determined using Pearson correlation coefficients. A P<0.05 was considered statistically significant.
Both ND- and HFD-fed rats exhibited decreased CRF levels and a decrease in expression of soluble-receptor for advanced glycation end product (sRAGE), an anti-aging marker (Fig. 1). Moreover, metabolic parameters and cardiac function deteriorated from baseline to follow-up in both groups of rats (details shown in Supplemental Table S1). These changes were more pronounced in HFD-fed rats than in ND-fed rats, and some parameters were altered only in HFD-fed rats, while those of ND-fed rats remained unchanged from baseline to follow-up (details shown in Supplemental Table S1). These results collectively support the proposal that obesity induces premature aging [12].
HFD-fed rats exhibited lower CRF levels and plasma sRAGE protein expression than found in ND-fed rats (Fig. 2). More unfavorable metabolic, cardiac, and brain parameters were observed in HFD-fed rats than in ND-fed rats (details shown in Supplemental Tables S2-S4, Supplemental Figs. S2, S3). Since these unfavorable profiles are also associated with aging [17-19], our results support the proposal that obesity results in premature metabolic, cardiac, and brain aging [12,20,21].
The relationship between CRF levels at baseline and at follow-up was investigated. There was a positive correlation between baseline CRF levels and CRF levels at follow-up in both ND-and HFD-fed rats (Fig. 3A), providing support that CRF is closely linked to genetics [2]. Interestingly, CRF showed a positive correlation with average food energy intake (Fig. 3B). This finding suggests that the effect of high CRF on protection against obesity is independent of calorie intake.
To determine the mechanisms underlying the protective effect of high CRF against metabolic dysfunction, we investigated the relationships of baseline CRF levels with metabolic parameters at follow-up and the absolute change of metabolic parameters from baseline to follow-up (value at week 28–value at week 12). We found that high baseline CRF levels were positively correlated with favorable metabolic parameters at follow-up, including favorable body weight and composition, increased wholebody substrate oxidation during exercise, decreased insulin resistance, and favorable lipid profiles (details shown in Fig. 4). The subgroup analyses also showed statistically significant differences in these parameters between rats with higher baseline CRF levels (ranked first to fourth in baseline running distance; n=4) and those with lower baseline CRF levels (ranked fifth to eighth in baseline running distance; n=4) within each diet group (details shown in Supplemental Table S5). Interestingly, the correlations between baseline CRF levels and metabolic parameters at follow-up were more obvious in HFD-fed rats than in ND-fed rats. Specifically, some metabolic parameters showed correlations with CRF levels at baseline only in HFD-fed rats, but not in ND-fed rats (details shown in Fig. 4). However, there were no significant correlations between changes in CRF levels from week 12 to 28 with metabolic parameters in the follow-up data. Importantly, the relationships between baseline CRF level and metabolic parameters were exhibited when the rats were only 35 weeks old, suggesting that high CRF protects against metabolic impairment starting in early life.
To determine the mechanisms responsible for the protective effect of high CRF against cardiac impairment, we identified the relationships between baseline CRF levels and cardiac parameters at follow-up. We also evaluated the relationships between baseline CRF levels and changes in cardiac function from baseline to follow-up. High CRF levels at baseline were positively correlated with favorable cardiac parameters at follow-up, including improved mitochondrial function, favorable mitochondrial metabolism, increased insulin signaling, increased mitochondrial respiration, decreased mitochondrial fission, increased mitochondrial fusion increased mitophagy, increased autophagy, decreased apoptosis, increased antioxidative capacity, decreased lipid peroxidation, decreased inflammation, increased anti-aging markers, and favorable cardiac function (details shown in Fig. 5, Supplemental Figs. S4, S5). The subgroup analyses also demonstrated significant or nearly-significant (P=0.05 to 0.09) differences in these parameters between rats with higher baseline CRF levels (ranked first to fourth in baseline running distance; n=4) and those with lower baseline CRF levels (ranked fifth to eighth in baseline running distance; n=4) within each diet group (details as shown in Supplemental Table S6). Specifically, the relationships between baseline CRF levels and cardiac parameters at follow-up were more obvious in HFD-fed rats than in ND-fed rats, since some cardiac parameters were only correlated with baseline CRF levels in HFD-fed rats, but not in ND-fed rats (details shown in Fig. 5, Supplemental Fig. S5). However, no significant correlations between changes in CRF levels from week 12 to 28 and cardiac parameters at follow-up were observed. Interestingly, the relationships between baseline CRF levels and cardiac parameters were displayed when the rats were only 35 weeks old, indicating that high CRF protects against cardiac impairment starting in an early period of life.
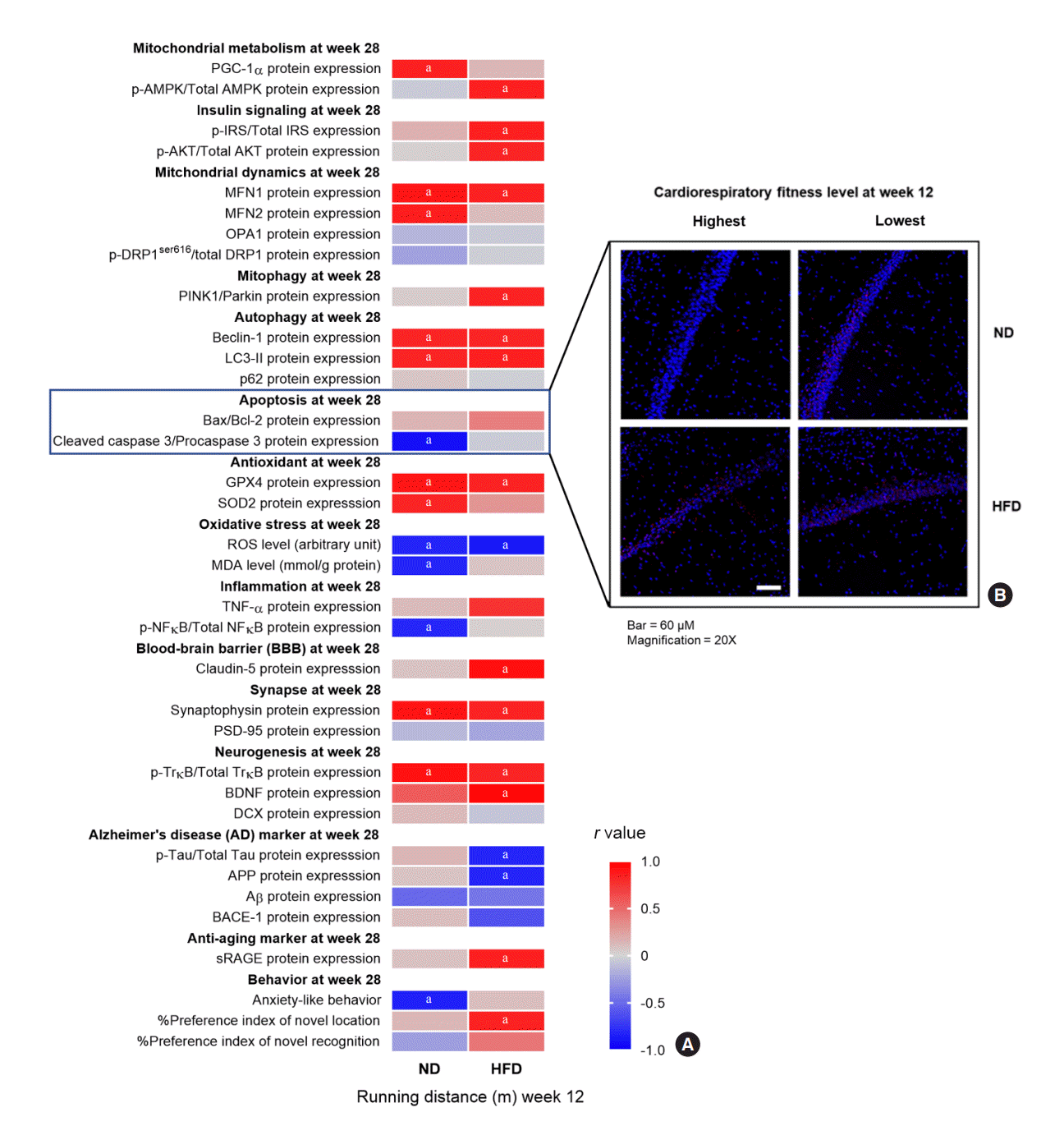
To determine the mechanisms underlying the protective effect of high CRF against brain impairment, we investigated the relationships between baseline CRF levels and brain parameters at follow-up. There were positive relationships between high baseline CRF levels and favorable brain parameters, including favorable metabolism, increased insulin signaling, decreased mitochondrial fission, increased mitochondrial fusion, increased mitophagy, increased autophagy, decreased apoptosis, increased antioxidative capacity, decreased oxidative stress, improved blood-brain barrier (BBB) function, improved synaptic function, increased neurogenesis, decreased AD markers, increased levels of an anti-aging marker, and improved cognition (details shown in Fig. 6, Supplemental Figs. S6, S7). The subgroup analyses also revealed a significant difference in these parameters between rats with higher baseline CRF levels (ranked first to fourth in baseline running distance; n=4) and those with lower baseline CRF levels (ranked fifth to eighth in baseline running distance; n=4) within each diet group (details shown in Supplemental Table S7). The relationships between baseline CRF levels and brain parameters at follow-up were more pronounced in HFD-fed rats than in ND-fed rats, as indicated by the findings that some brain parameters showed correlations with baseline CRF levels only in HFD-fed rats, but not in ND-fed rats (details shown in Fig. 6, Supplemental Fig. S7). Nevertheless, no significant correlations were found between changes in CRF levels from week 12 to 28 and brain parameters at follow-up. Importantly, the relationships between baseline CRF levels and brain parameters were exhibited when the rats were only 35 weeks old, suggesting that high CRF protects against brain impairment starting at an early stage of life.
In the present study, baseline CRF levels showed correlations with metabolic, cardiac, and brain parameters at the point of follow-up in both ND- and HFD-fed rats. We also found that HFD-fed rats exhibited aging-related unfavorable metabolic, cardiac, and brain profiles at follow-up, when compared with those of ND-fed rats. Taken together, it can be postulated that high CRF protects against metabolic, cardiac, and brain impairments both in normal conditions and in obesity-induced premature aging. Interestingly, the effect of high CRF as a protective mechanism against these impairments remained significant even though the follow-up duration was short (16 weeks) and the age of rats at follow-up was only 35 weeks (equivalent to less than 30 years in humans) [22], suggesting that the impact of CRF begins early in life.
We found significant correlations between baseline CRF levels and a variety of metabolic parameters at the time of follow-up, suggesting that high CRF delays metabolic impairment via the amelioration of obesity, impaired carbohydrate and fatty acid oxidation during vigorous exercise, reduced insulin resistance, and dyslipidemia. All of these findings have been reported in previous studies using HCR and LCR rats [7,8,11]. However, in our study, using non-selectively bred rats with a short duration of follow-up, it was found that metabolic parameters were significantly affected by CRF and the consequences of high CRF occurred early in life. The parameters that CRF appeared to have significantly impacted were body weight, visceral fat weight, fasting glucose, carbohydrate oxidation, triglyceride levels, and total cholesterol levels, as the correlations between baseline CRF levels and these parameters at follow-up were present in both ND and HFD-fed rats. Additionally, a comparison of the effect of high CRF against metabolic impairment between ND and HFD-fed rats demonstrated that this effect was stronger in obesity-induced premature aging. These findings support the proposal that high CRF confers protection against HFD-induced metabolic syndrome [23,24].
Our results showed relationships between baseline CRF levels and several cardiac parameters at the time of follow-up. These parameters could reflect the potential for several mechanisms to underlie the protective effect of high CRF against cardiac impairment, including mitochondrial function, metabolism, mitochondrial respiration, insulin signaling, mitochondrial dynamics, mitophagy, autophagy, apoptosis, redox reaction, inflammation, and systolic function. Interestingly, the effect of high CRF on protection against cardiac impairment was stronger in obesity-induced premature aging. Unlike some previous studies in HCR and LCR rats [13,14], we did not observe a relationship between baseline CRF levels and diastolic function at follow-up in normal and obese rats, and a relationship between baseline CRF levels and systolic function at follow-up was also not exhibited in normal rats. The contradictory results may be explained by the short follow-up in our study, for which reason the rats did not reach the age classified as elderly, when these correlations may have been exhibited. Nonetheless, our study, using non-selectively bred rats with a short follow-up, identified the cardiac parameters that were significantly affected by CRF levels early in life. These parameters were mitochondrial aspects, insulin signaling, and apoptosis, for which correlations with baseline CRF levels were observed in both ND- and HFD-fed rats.
We also observed relationships between baseline CRF levels and several brain parameters at follow-up, which may be mechanistically related to the protective effect of high CRF against brain impairment. These mechanisms included metabolism, insulin signaling, mitochondrial fusion, mitophagy, autophagy, apoptosis, redox reactions, inflammation, BBB, synaptic function, neurogenesis, AD markers, an anti-aging marker, anxiety-like behavior, and learning behavior. Consistent with metabolic and cardiac parameters, the protective effect of high CRF against brain impairment was stronger in obesity-induced premature aging. Since there were relationships between baseline CRF levels and mitochondrial aspects, autophagy, redox reactions, synaptic function, and neurogenesis in both ND and HFD-fed rats at follow-up, these brain parameters are considered to be closely affected by the level of CRF, particularly in early life.
First of all, this study cannot explain why the protective effect of high CRF against metabolic, cardiac, and brain impairment was stronger in obesity-induced premature aging. To further develop this line of inquiry, an additional molecular study comparing metabolic, cardiac, and brain profiles between normal and obese animals with the same level of baseline CRF would be needed. In addition, this study used an obesity-induced premature aging model that may not be representative of other premature aging models; hence, the findings may not be transferable. For that reason, a further study with various models of premature aging would be useful to determine the protective effect of high CRF against different pathological conditions. Additionally, we included only male rats in this study to control for sex; however, it is widely accepted that sex hormones are critical in determining lean body mass [25,26], and consequently CRF levels [27]. Hence, the findings of our study may not be transferable to clinical practice for female patients.
In conclusion, the protective effect of high CRF against metabolic, cardiac, and brain impairments was stronger in obesity-induced premature aging than in normal conditions. These findings imply that high CRF is a crucial protective factor against metabolic syndrome, cardiovascular diseases, and neurodegenerative disorders, especially in conditions of obesity. Moreover, because our molecular study indicated that these effects of CRF began at a relatively young age, high CRF might exhibit early protection against metabolic, cardiac, and brain impairments via various molecular mechanisms. Taken together, any interventions that help increase CRF levels starting in an early period of life would be considered highly beneficial, specifically in obese individuals. Several studies have reported that CRF was increased after exercise training [28-31]. Therefore, our findings emphasize the benefits of exercise training on metabolic, cardiac and brain health conditions.
ACKNOWLEDGMENTS
This study was supported by the Faculty of Medicine Chiang Mai University Endowment Fund: 060-2564 (Chanisa Thonusin); a Senior Research Scholar Grant from the National Research Council of Thailand (Siriporn C. Chattipakorn); a Teaching Assistant and Research Assistant (TARA) Scholarship, Chiang Mai University, Thailand (Patcharapong Pantiya); the Royal Golden Jubilee Ph.D. program from the National Research Council of Thailand (N41A650088: Patcharapong Pantiya & Siriporn C. Chattipakorn); the Research Grant for New Scholar from the National Research Council of Thailand (RGNS 64-059 Chanisa Thonusin); the Royal Golden Jubilee Ph.D. program (PHD/0106/2561 Benjamin Ongnok & Siriporn C. Chattipakorn); the NSTDA Research Chair Grant from the National Science and Technology Development Agency Thailand (Nipon Chattipakorn); a Chiang Mai University Center of Excellence Award (Nipon Chattipakorn); and the Thailand Science Research and Innovation grant DBG6280006 (Nipon Chattipakorn).
Notes
AUTHOR CONTRIBUTION
Conception or design: P.P., C.T., N.C., S.C.C. Acquisition, analysis, or interpretation of data: P.P., C.T., N.S., B.O., T.C., S.K., T.J., B.A., N.S.A., S.S., N.C., S.C.C. Drafting the work or revising: P.P., C.T., N.C., S.C.C. Final approval of the manuscript: P.P., C.T., N.C., S.C.C.
Supplementary Information
Supplemental Table S1.
Metabolic Parameters and Cardiac Function at Baseline (Week 12) versus Follow-up (Week 28)
Supplemental Table S2.
Metabolic Parameters in ND-Fed Rats versus HFD-Fed Rats
Supplemental Table S3.
Cardiac Parameters in ND-Fed Rats versus HFD-Fed Rats
Supplemental Table S5.
Baseline Running Distance, Anthropometry, Whole-Body Substrate Oxidation, and Metabolic Parameters at Follow-up between Higher versus Lower Baseline CRF Subgroup
Supplemental Table S6.
Cardiac Parameters at Follow-up between Rats with Higher Baseline CRF versus Rats with Lower Baseline CRF
Supplemental Table S7.
Brain Parameters at Follow-up between Rats with Higher Baseline CRF versus Rats with Lower Baseline CRF
Supplemental Fig. S1.
The experimental protocol of the study. OFT, open-field test; NOL, novel object location; NOR, novel object recognition; CRF, cardiorespiratory fitness; HRV, heart rate variability.
Supplemental Fig. S2.
Representative picture of Western blotting in cardiac tissue of normal diet-fed rats (ND) versus high-fat diet-fed rats (HFD) at follow-up (week 28). CPT1, carnitine palmitoyltransferase I; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1α; p-AMPK, phosphorylated-activated protein kinase; AMPK, activated protein kinase; p-IRS, phosphorylated-insulin receptor substrate 1; IRS, insulin receptor substrate 1; MFN1, mitofusin 1; MFN2, mitofusin 2; OPA1, optic atrophy 1; DRP1, dynamin-related protein 1; p-DRP1ser616, phosphorylated-dynamin-related at serine616; PINK1, PTEN-induced kinase 1; LC3-II, light chain 3-II; Bax, Bcl-2-associated X protein; Bcl, B-cell lymphoma; GPX4, glutathione peroxidase 4; SOD2, superoxide dismutase 2; MDA, malondialdehyde; TNF-α, tumor necrosis factor-α; p-NFκB, phosphorylated-nuclear factor kappa-light-chain-enhancer of activated B cells; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; sRAGE, soluble-receptor for advanced glycation end product; VDAC, voltage-dependent anion channel; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Supplemental Fig. S3.
Representative picture of Western blotting in the brain tissue of normal diet-fed rats (ND) versus high-fat diet-fed rats (HFD) at follow-up (week 28). PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1α; p-AMPK, phosphorylated-activated protein kinase; AMPK, activated protein kinase; p-IRS, phosphorylated-insulin receptor substrate 1; IRS, insulin receptor substrate 1; MFN1, mitofusin 1; MFN2, mitofusin 2; OPA1, optic atrophy 1; p-DRP1ser616, phosphorylated-dynamin-related at serine616; DRP1, dynamin-related protein 1; PINK1, PTEN-induced kinase 1; LC3-II, light chain 3-II; Bax/Bcl, Bcl-2-associated X protein/B-cell lymphoma; GPX4, glutathione peroxidase 4; SOD2, superoxide dismutase 2; TNF-α, tumor necrosis factor-α; p-NFκB, phosphorylated-nuclear factor kappa-light-chain-enhancer of activated B cells; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; PSD-95, postsynaptic density protein 95; p-TrκB, phosphorylated-tropomyosin receptor kinase B; TrκB, tropomyosin receptor kinase B; BDNF, brain-derived neurotrophic factor; DCX, doublecortin; p-Tau, phosphorylated-Tau; APP, amyloid-beta precursor protein; Aβ, amyloid β; BACE-1, beta-site amyloid precursor protein cleaving enzyme 1; sRAGE, soluble-receptor for advanced glycation end product.
Supplemental Fig. S4.
Full Western blot for cardiac protein expressions. CPT1, carnitine palmitoyltransferase I; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1α; p-DRP1, phosphorylated-dynamin-related protein 1; DRP1, dynamin-related protein 1; p-AMPK, phosphorylated-activated protein kinase; AMPK, activated protein kinase; PINK1, PTEN-induced kinase 1; p-NFκB, phosphorylated-nuclear factor kappa-light-chain-enhancer of activated B cells; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; p-IRS, phosphorylated-insulin receptor substrate 1; IRS, insulin receptor substrate 1; TNF-α, tumor necrosis factor-α; VDAC, voltage-dependent anion channel; LC3-II, light chain 3-II; sRAGE, soluble-receptor for advanced glycation end product; ND, normal diet-fed rats; HFD, high-fat diet-fed rats; MFN1, mitofusin 1; GPX4, glutathione peroxidase 4; MFN2, mitofusin 2; SOD2, superoxide dismutase 2; OPA1, optic atrophy 1; Bax, Bcl-2-associated X protein; Bcl, B-cell lymphoma; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Supplemental Fig. S5.
The representatives of Western blotting data for cardiac protein expressions between a rat with highest cardiorespiratory fitness (CRF) level versus a rat with lowest CRF level of each group. ND, normal diet; HFD, high-fat diet; CPT1, carnitine palmitoyltransferase I; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1α; p-AMPK, phosphorylated-activated protein kinase; p-IRS, phosphorylated-insulin receptor substrate 1; IRS, insulin receptor substrate 1; MFN1, mitofusin 1; MFN2, mitofusin 2; OPA1, optic atrophy 1; DRP1, dynamin-related protein 1; p-DRP1ser616, phosphorylated-dynamin-related at serine616; PINK1, PTEN-induced kinase 1; LC3-II, light chain 3-II; Bax, Bcl-2-associated X protein; Bcl, B-cell lymphoma; GPX4, glutathione peroxidase 4; SOD2, superoxide dismutase 2; TNF-α, tumor necrosis factor-α; p-NFκB, phosphorylated-nuclear factor kappa-light-chain-enhancer of activated B cells; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; sRAGE, soluble-receptor for advanced glycation end product; VDAC, voltage-dependent anion channel; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Supplemental Fig. S6.
Full Western blot for brain protein expressions. p-AMPK, phosphorylated-activated protein kinase; AMPK, activated protein kinase; LC3-II, light chain 3-II; PSD-95, postsynaptic density protein 95; APP, amyloid-beta precursor protein; p-IRS, phosphorylated-insulin receptor substrate 1; IRS, insulin receptor substrate 1; GPX4, glutathione peroxidase 4; p-Tau, phosphorylated-Tau; SOD2, superoxide dismutase 2; TNF-α, tumor necrosis factor-α; Aβ, amyloid β; BACE-1, beta-site amyloid precursor protein cleaving enzyme 1; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1α; MFN1, mitofusin 1; Bax, Bcl-2-associated X protein; Bcl, B-cell lymphoma; BDNF, brain-derived neurotrophic factor; DCX, doublecortin; MFN2, mitofusin 2; OPA1, optic atrophy 1; p-NFκB, phosphorylated-nuclear factor kappa-light-chain-enhancer of activated B cells; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; p-TrκB, phosphorylated-tropomyosin receptor kinase B; TrκB, tropomyosin receptor kinase B; p-DRP1, phosphorylated-dynamin-related protein 1; DRP1, dynamin-related protein 1; sRAGE, soluble-receptor for advanced glycation end product; PINK1, PTEN-induced kinase 1; ND, normal diet-fed rats; HFD, high-fat diet-fed rats.
Supplemental Fig. S7.
The representatives of Western blotting data for brain protein expressions between a rat with highest cardiorespiratory fitness (CRF) level versus a rat with lowest CRF level of each group. ND, normal diet; HFD, high-fat diet; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1α; p-AMPK, phosphorylated-activated protein kinase; p-IRS, phosphorylated-insulin receptor substrate 1; IRS, insulin receptor substrate 1; MFN1, mitofusin 1; MFN2, mitofusin 2; OPA1, optic atrophy 1; p-DRP1ser616, phosphorylated-dynamin-related at serine616; DRP1, dynamin-related protein 1; PINK1, PTEN-induced kinase 1; LC3-II, light chain 3-II; Bax, Bcl-2-associated X protein; Bcl, B-cell lymphoma; GPX4, glutathione peroxidase 4; SOD2, superoxide dismutase 2; TNF-α, tumor necrosis factor-α; p-NFκB, phosphorylated-nuclear factor kappa-light-chain-enhancer of activated B cells; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; PSD-95, postsynaptic density protein 95; p-TrκB, phosphorylated-tropomyosin receptor kinase B; TrκB, tropomyosin receptor kinase B; BDNF, brain-derived neurotrophic factor; DCX, doublecortin; p-Tau, phosphorylated Tau; APP, amyloid-beta precursor protein; Aβ, amyloid β; BACE-1, beta-site amyloid precursor protein cleaving enzyme 1; sRAGE, soluble-receptor for advanced glycation end product.
REFERENCES
1. Lee DC, Artero EG, Sui X, Blair SN. Mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol. 2010; 24(4 Suppl):27–35.

2. Schutte NM, Nederend I, Hudziak JJ, Bartels M, de Geus EJ. Twin-sibling study and meta-analysis on the heritability of maximal oxygen consumption. Physiol Genomics. 2016; 48:210–9.

3. Vidoni ED, Honea RA, Billinger SA, Swerdlow RH, Burns JM. Cardiorespiratory fitness is associated with atrophy in Alzheimer’s and aging over 2 years. Neurobiol Aging. 2012; 33:1624–32.

4. Liu R, Sui X, Laditka JN, Church TS, Colabianchi N, Hussey J, et al. Cardiorespiratory fitness as a predictor of dementia mortality in men and women. Med Sci Sports Exerc. 2012; 44:253–9.

5. Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009; 301:2024–35.

6. Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001; 5:45–52.

7. Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005; 307:418–20.

8. Rivas DA, Lessard SJ, Saito M, Friedhuber AM, Koch LG, Britton SL, et al. Low intrinsic running capacity is associated with reduced skeletal muscle substrate oxidation and lower mitochondrial content in white skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2011; 300:R835–43.

9. Crissey JM, Padilla J, Vieira-Potter VJ, Thorne PK, Koch LG, Britton SL, et al. Divergent role of nitric oxide in insulin-stimulated aortic vasorelaxation between low- and high-intrinsic aerobic capacity rats. Physiol Rep. 2015; 3:e12459.

10. Gavini CK, Mukherjee S, Shukla C, Britton SL, Koch LG, Shi H, et al. Leanness and heightened nonresting energy expenditure: role of skeletal muscle activity thermogenesis. Am J Physiol Endocrinol Metab. 2014; 306:E635–47.

11. Overmyer KA, Evans CR, Qi NR, Minogue CE, Carson JJ, Chermside-Scabbo CJ, et al. Maximal oxidative capacity during exercise is associated with skeletal muscle fuel selection and dynamic changes in mitochondrial protein acetylation. Cell Metab. 2015; 21:468–78.

12. Salvestrini V, Sell C, Lorenzini A. Obesity may accelerate the aging process. Front Endocrinol (Lausanne). 2019; 10:266.

13. Hussain SO, Barbato JC, Koch LG, Metting PJ, Britton SL. Cardiac function in rats selectively bred for low- and high-capacity running. Am J Physiol Regul Integr Comp Physiol. 2001; 281:R1787–91.

14. Koch LG, Kemi OJ, Qi N, Leng SX, Bijma P, Gilligan LJ, et al. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res. 2011; 109:1162–72.

15. Burghardt PR, Flagel SB, Burghardt KJ, Britton SL, GerardKoch L, Watson SJ, et al. Risk-assessment and coping strategies segregate with divergent intrinsic aerobic capacity in rats. Neuropsychopharmacology. 2011; 36:390–401.

16. Choi J, Chandrasekaran K, Demarest TG, Kristian T, Xu S, Vijaykumar K, et al. Brain diabetic neurodegeneration segregates with low intrinsic aerobic capacity. Ann Clin Transl Neurol. 2014; 1:589–604.

17. Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res. 2012; 110:1109–24.

18. Bonomini F, Rodella LF, Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015; 6:109–20.

19. Grimm A, Eckert A. Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochem. 2017; 143:418–31.

20. Thorpe RJ Jr, Ferraro KF. Aging, obesity, and mortality: misplaced concern about obese older people? Res Aging. 2004; 26:108–29.
21. Tam BT, Morais JA, Santosa S. Obesity and ageing: two sides of the same coin. Obes Rev. 2020; 21:e12991.

22. Sengupta P. The laboratory rat: relating its age with human’s. Int J Prev Med. 2013; 4:624–30.
23. Morris EM, Jackman MR, Johnson GC, Liu TW, Lopez JL, Kearney ML, et al. Intrinsic aerobic capacity impacts susceptibility to acute high-fat diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab. 2014; 307:E355–64.
24. Thyfault JP, Morris EM. Intrinsic (genetic) aerobic fitness impacts susceptibility for metabolic disease. Exerc Sport Sci Rev. 2017; 45:7–15.

25. Harman SM, Blackman MR. The effects of growth hormone and sex steroid on lean body mass, fat mass, muscle strength, cardiovascular endurance and adverse events in healthy elderly women and men. Horm Res. 2003; 60(Suppl 1):121–4.

26. Brown M. Skeletal muscle and bone: effect of sex steroids and aging. Adv Physiol Educ. 2008; 32:120–6.

27. Zeiher J, Ombrellaro KJ, Perumal N, Keil T, Mensink G, Finger JD. Correlates and determinants of cardiorespiratory fitness in adults: a systematic review. Sports Med Open. 2019; 5:39.

28. Chockalingam A, Linden MA, Del Rosario M, Govindarajan G, Dellsperger KC, Thomas TR. Exercise and weight loss improve exercise capacity independent of cardiac function in metabolic syndrome. Angiology. 2010; 61:192–7.

29. Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC, et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2015; 4:e002014.

30. Frimpong E, Dafkin C, Donaldson J, Millen A, Meiring RM. The effect of home-based low-volume, high-intensity interval training on cardiorespiratory fitness, body composition and cardiometabolic health in women of normal body mass and those with overweight or obesity: protocol for a randomized controlled trial. BMC Sports Sci Med Rehabil. 2019; 11:39.

31. American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998; 30:975–91.
Fig. 1.
Cardiorespiratory fitness (CRF) and plasma soluble-receptor for advanced glycation end product (sRAGE) protein expression at baseline (week 12) versus follow-up (week 28). (A) CRF, (B) plasma sRAGE protein expression (n=8 per group). The CRF level is reported as running distance. Data are reported as mean±standard error of the mean (SEM). The values on the top of each bar represent mean±SEM values of CRF levels. ND, normal diet; HFD, high-fat diet. aP<0.05 when compared to baseline within the same group (week 12).
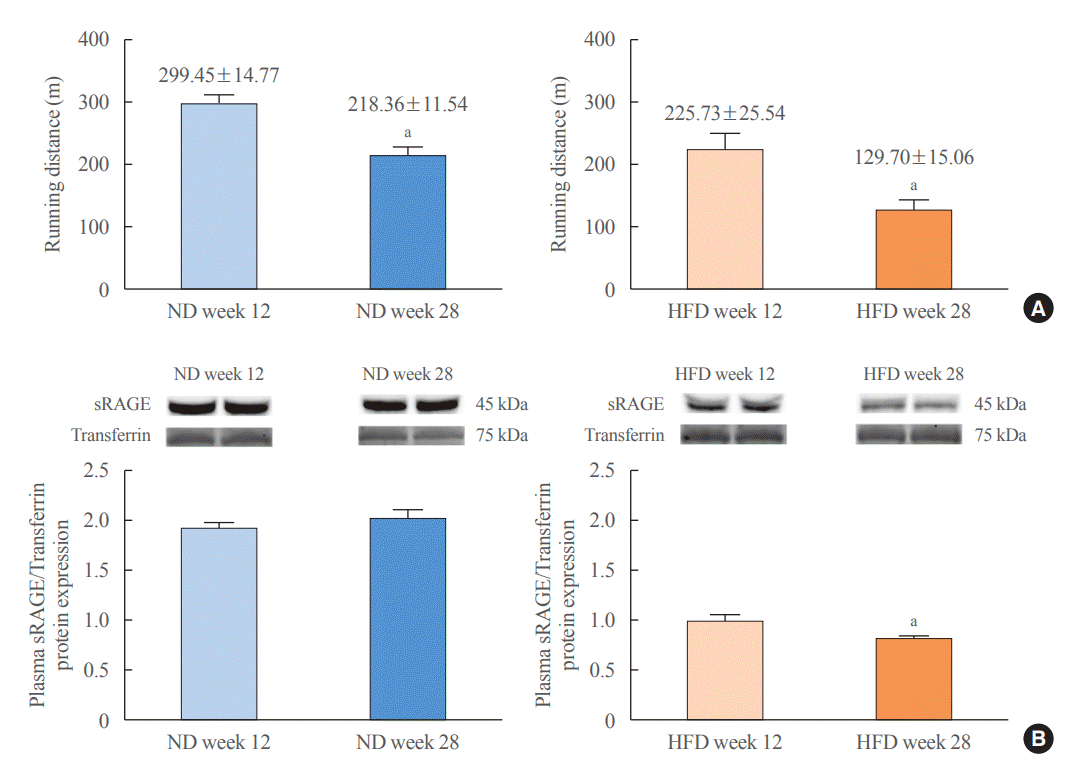
Fig. 2.
Cardiorespiratory fitness (CRF) and plasma soluble-receptor for advanced glycation end product (sRAGE) protein expression in normal diet (ND)-fed rats versus high-fat diet (HFD)-fed rats. (A) CRF, (B) plasma sRAGE protein expression (n=8 per group; week 12=baseline; week 28=follow-up). The CRF level is reported as running distance. Data are reported as mean±standard error of the mean (SEM). The values on the top of each bar represent mean±SEM values of CRF levels. aP<0.05 when compared to ND-fed rats at the same time point.
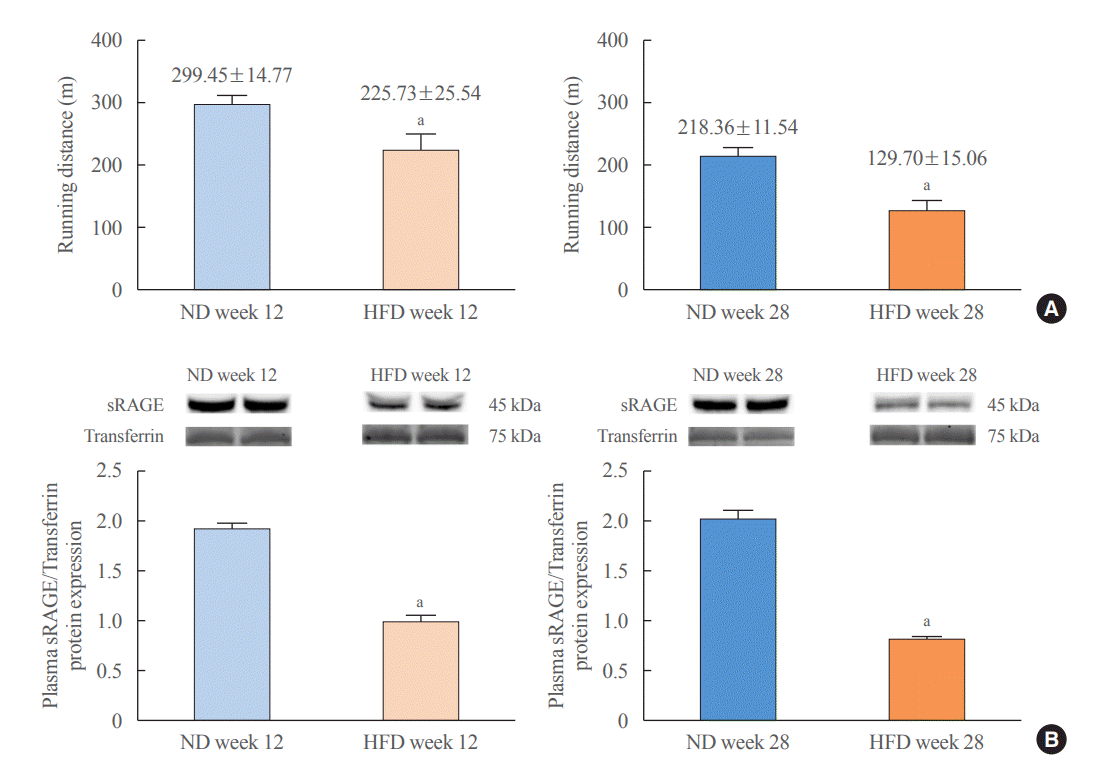
Fig. 3.
Cardiorespiratory fitness (CRF) at baseline is positively correlated with CRF at follow-up and food intake. The scatter plots display (A) the correlation between CRF at baseline (week 12) and follow-up (week 28), and (B) the correlation between CRF at baseline (week 12) and average food intake (B) (n=8 per group). The CRF level is reported as running distance. Data are reported as r values. ND, normal diet; HFD, high-fat diet. aP<0.05.
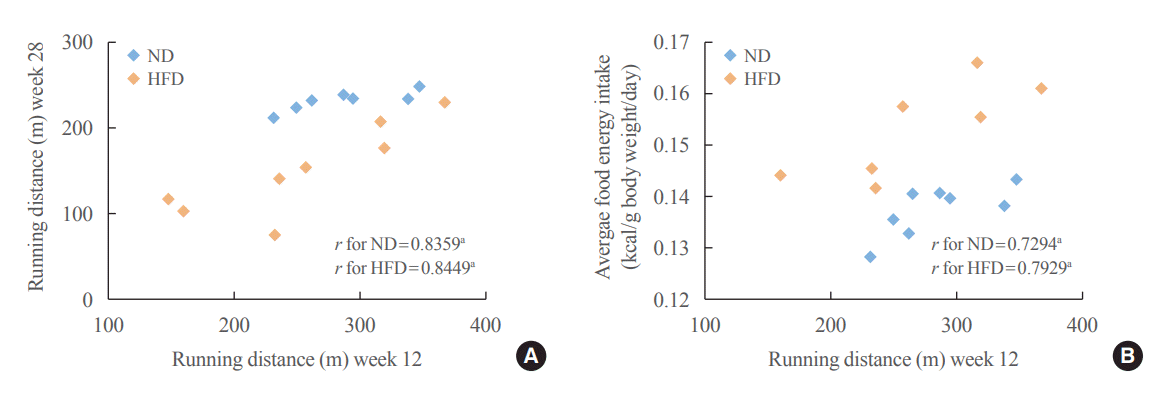
Fig. 4.
The effects of cardiorespiratory fitness (CRF) at baseline on metabolic parameters. Heatmap displaying correlations between CRF at baseline (week 12) and metabolic parameters at follow-up (week 28); correlations between CRF at baseline (week 12) and the absolute change (Δ) of metabolic parameters from baseline to follow-up (value at week 28–value at week 12) (n=8 per group). The CRF level is reported as running distance. Data are reported as r values. FAO, fatty acid oxidation rate; CHOO, carbohydrate oxidation rate; HOMA-IR, homeostatic model assessment for insulin resistance; HDL, high density lipoprotein; LDL, low density lipoprotein; ND, normal diet; HFD, high-fat diet. aP<0.05.
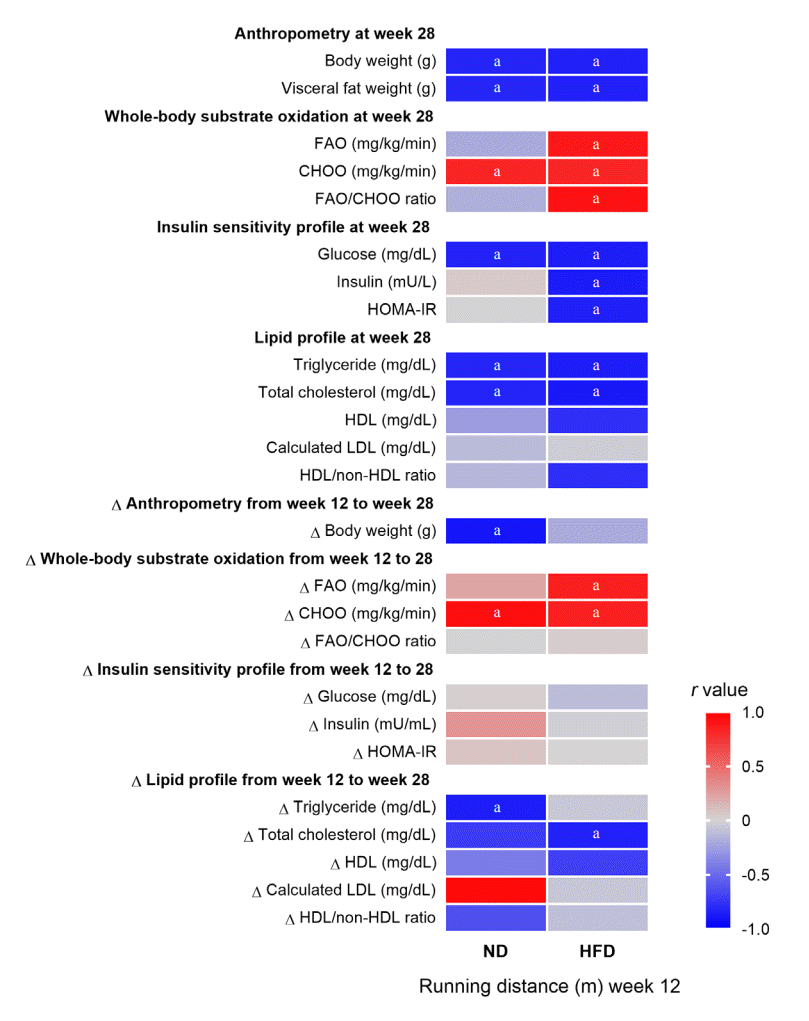
Fig. 5.
The effects of cardiorespiratory fitness (CRF) at baseline on cardiac parameters. (A) Heatmap displaying: correlations between CRF at baseline (week 12) and cardiac parameters at follow-up (week 28); correlations between CRF at baseline (week 12) and the absolute change (Δ) of cardiac function from baseline to follow-up (value at week 28–value at week 12). (B) Representative pictures of apoptotic cell death in left ventricular tissue (n=8 per group). The CRF level is reported as running distance. Data are reported as r values. ROS, reactive oxygen species; CPT1, carnitine palmitoyltransferase I; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1α; p-AMPK, phosphorylated-activated protein kinase; AMPK, activated protein kinase; p-IRS, phosphorylated-insulin receptor substrate 1; IRS, insulin receptor substrate 1; MFN1, mitofusin 1; MFN2, mitofusin 2; OPA1, optic atrophy 1; p-DRP1ser616, phosphorylated-dynamin-related at serine616; DRP1, dynamin-related protein 1; PINK1, PTEN-induced kinase 1; LC3-II, light chain 3-II; Bax/Bcl, Bcl-2-associated X protein/B-cell lymphoma; GPX4, glutathione peroxidase 4; SOD2, superoxide dismutase 2; MDA, malondialdehyde; TNF-α, tumor necrosis factor-α; p-NFκB, phosphorylated-nuclear factor kappa-light-chain-enhancer of activated B cells; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; sRAGE, soluble-receptor for advanced glycation end product; LVEF, left ventricular ejection fraction; FS, fractional shortening; E/A, early to late ventricular filling velocity; LF/HF, lower frequency/high frequency; ND, normal diet; HFD, high-fat diet. aP<0.05.
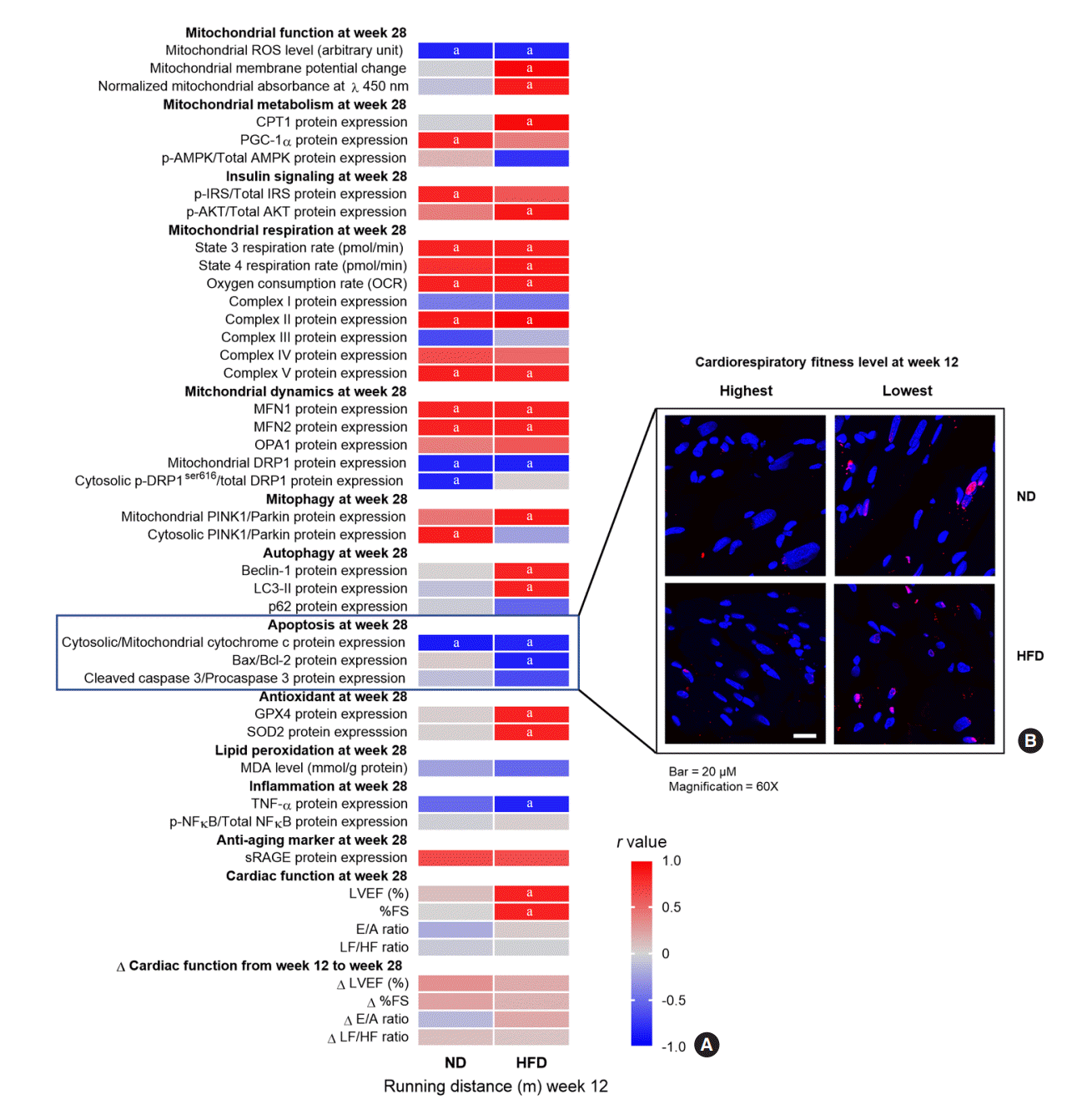
Fig. 6.
The effects of cardiorespiratory fitness (CRF) at baseline on brain parameters. (A) Heatmap displaying correlations between CRF at baseline (week 12) and brain parameters at follow-up (week 28). (B) Representative pictures of apoptotic cell death at CA1 of the hippocampus (n=8 per group). The CRF level is reported as running distance. Data are reported as r values. PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1α; p-AMPK, phosphorylated-activated protein kinase; AMPK, activated protein kinase; MFN1, mitofusin 1; MFN2, mitofusin 2; OPA1, optic atrophy 1; p-DRP1ser616, phosphorylated-dynamin-related at serine616; DRP1, dynamin-related protein 1; PINK1, PTEN-induced kinase 1; LC3-II, light chain 3-II; Bax/Bcl, Bcl-2-associated X protein/B-cell lymphoma; GPX4, glutathione peroxidase 4; SOD2, superoxide dismutase 2; ROS, reactive oxygen species; MDA, malondialdehyde; TNF-α, tumor necrosis factor-α; p-NFκB, phosphorylated-nuclear factor kappa-light-chain-enhancer of activated B cells; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; PSD-95, postsynaptic density protein 95; p-TrκB, phosphorylated-tropomyosin receptor kinase B; TrκB, tropomyosin receptor kinase B; BDNF, brain-derived neurotrophic factor; DCX, doublecortin; p-Tau, phosphorylated-Tau; APP, amyloid-beta precursor protein; Aβ, amyloid β; BACE-1, beta-site amyloid precursor protein cleaving enzyme 1; sRAGE, soluble-receptor for advanced glycation end product; ND, normal diet; HFD, high-fat diet. aP<0.05.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download