Abstract
Background
Methods
Results
Supplementary Information
Supplemental Table S2.
Supplemental Table S3.
Supplemental Table S4.
Supplemental Fig. S1.
ACKNOWLEDGMENTS
REFERENCES
Fig. 1
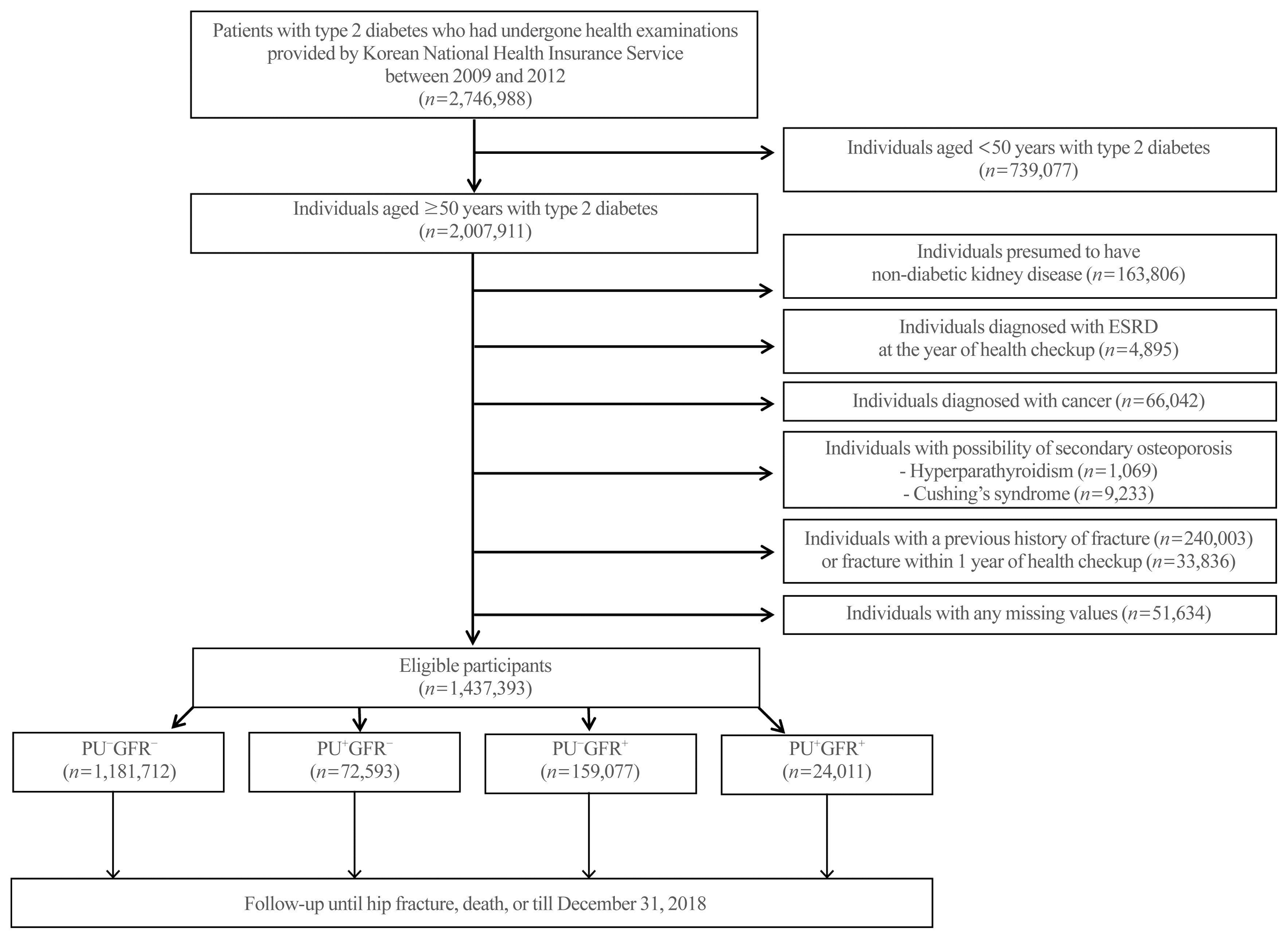
Fig. 2
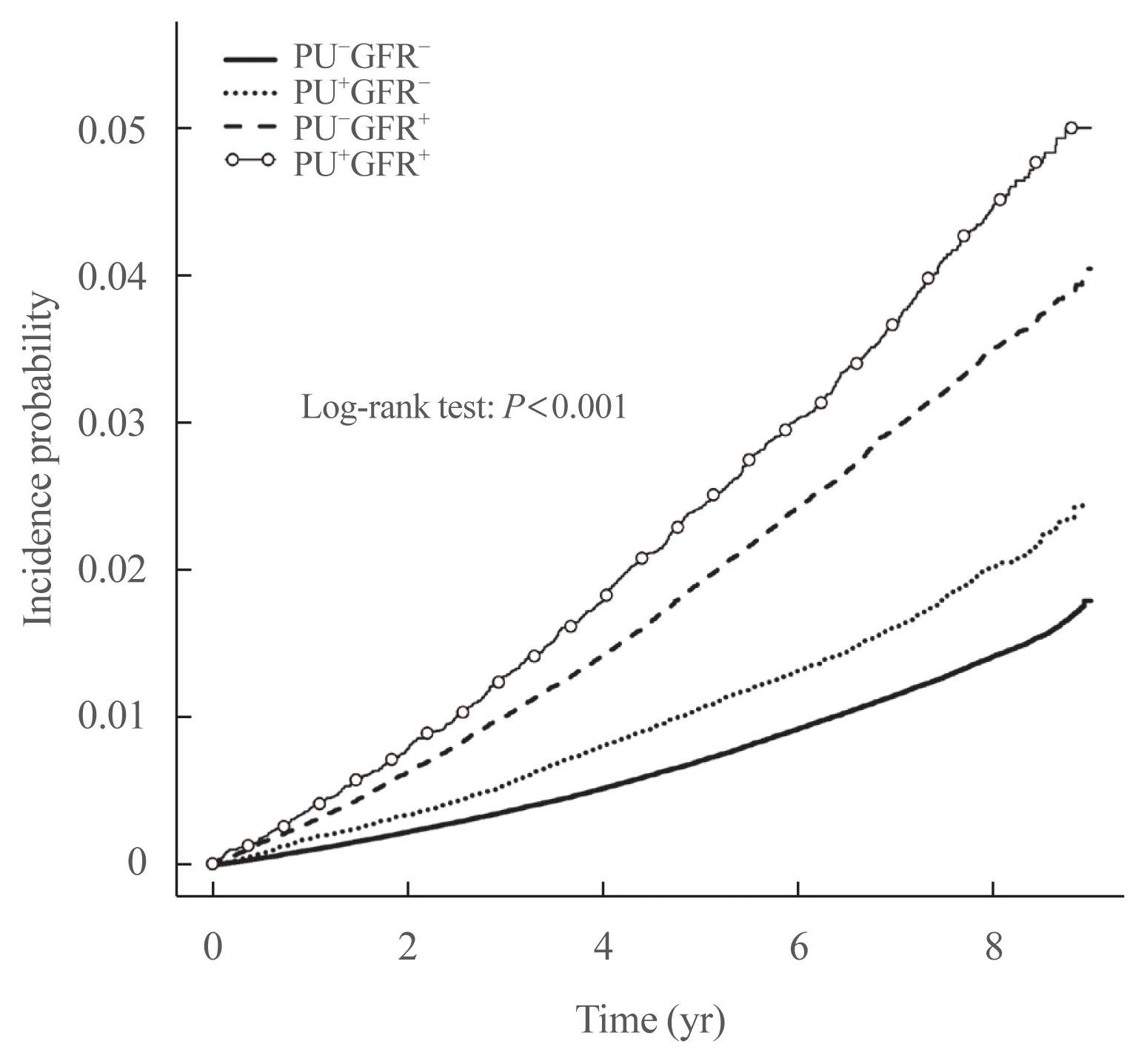
Fig. 3
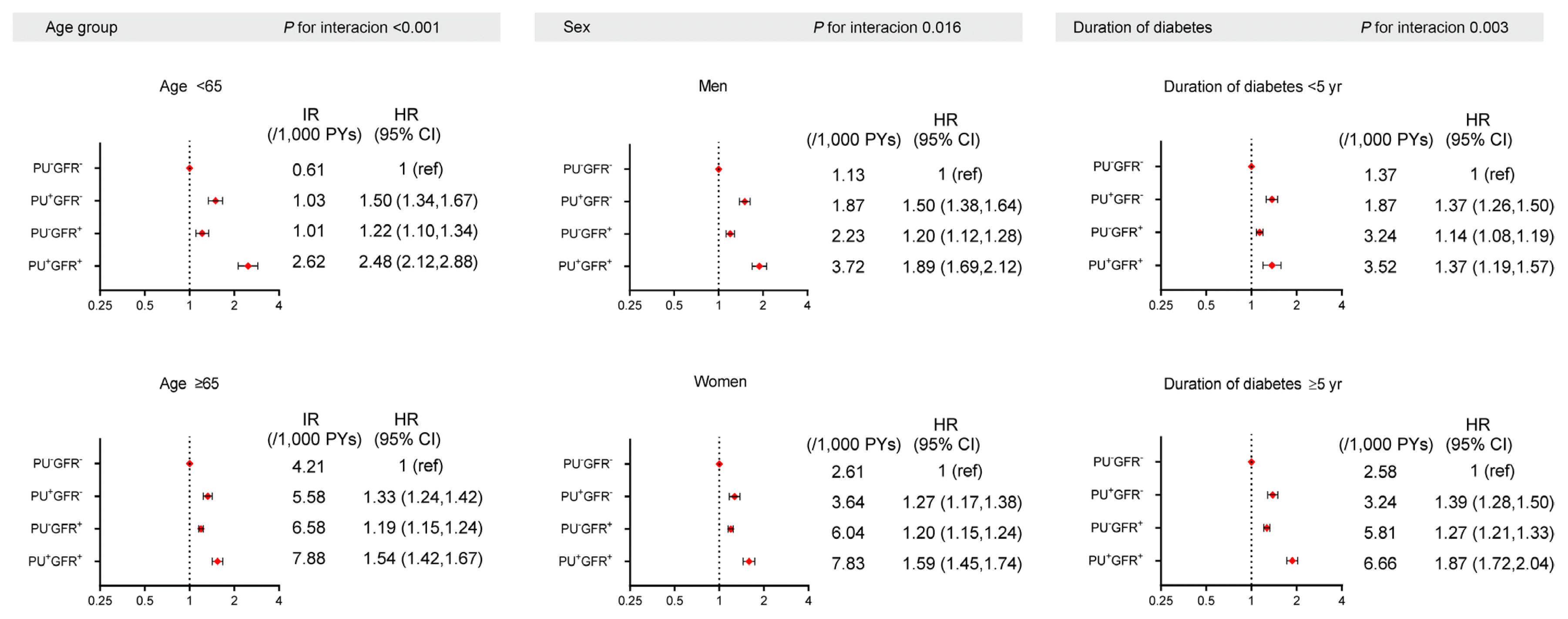
Table 1
| Characteristic | PU−GFR− (n=1,181,712) | PU+GFR− (n=72,593) | PU−GFR+ (n=159,077) | PU+GFR+ (n=24,011) | P value |
|---|---|---|---|---|---|
| Age, yr | 61.3±8.1c,d | 61.4±8.2c,d | 67.3±8.5a,b,d | 66.1±8.5a,b,c | <0.001 |
|
|
|||||
| Female sex | 483,376 (40.9)b,c | 23,478 (32.3)a,c,d | 87,177 (54.8)a,b,d | 9,698 (40.4)b,c | <0.001 |
|
|
|||||
| BMI, kg/m2 | 24.9±3.2b,c,d | 25.2±3.4a | 25.1±3.3a | 25.1±3.4a | <0.001 |
|
|
|||||
| Current smoker | 263,180 (22.3)b,c,d | 19,837 (27.3)a,c,d | 21,260 (13.4)a,b,d | 4,359 (18.2)a,b,c | <0.001 |
|
|
|||||
| Heavy drinker | 109,138 (9.2)b,c,d | 9,313 (12.8)a,c,d | 6,710 (4.2)a,b,d | 1,347 (5.6)a,b,c | <0.001 |
|
|
|||||
| Comorbidities | |||||
| Hypertension | 703,802 (59.6)b,c,d | 53,010 (73.0)a,c,d | 120,203 (75.6)a,b,d | 20,991 (87.4)a,b,c | <0.001 |
| Dyslipidemia | 487,680 (41.3)b,c,d | 35,272 (48.6)a,c,d | 79,759 (50.1)a,b,d | 13,931 (58.0)a,b,c | <0.001 |
| Ischemic heart disease | 211,565 (17.9)b,c,d | 14,586 (20.1)a,c,d | 43,738 (27.5)a,b,d | 7,560 (31.5)a,b,c | <0.001 |
| Stroke | 83,972 (7.1)b,c,d | 6,328 (8.7)a,c,d | 21,379 (13.4)a,b,d | 3,977 (16.6)a,b,c | <0.001 |
| Heart failure | 30,020 (2.5)b,c,d | 2,436 (3.4)a,c,d | 10,374 (6.5)a,b,d | 1,847 (7.7)a,b,c | <0.001 |
|
|
|||||
| Severity of diabetes | |||||
| Duration of diabetes ≥5 yr | 377,126 (31.9)b,c,d | 32,080 (44.2)a,d | 70,180 (44.1)a,d | 15,129 (63.0)a,b,c | <0.001 |
| Insulin | 88,115 (7.5)b,c,d | 10,627 (14.6)a,c,d | 20,562 (12.9)a,b,d | 70,29 (29.3)a,b,c | <0.001 |
| OHA ≥2 classes | 49,6450 (42.0)b,c,d | 38,903 (53.6)a,c,d | 78,178 (49.1)a,b,d | 13,755 (57.3)a,b,c | <0.001 |
| Proliferative DR | 5,634 (0.5)b,c,d | 1,362 (1.9)a,c,d | 1,449 (0.9)a,b,d | 969 (4.0)a,b,c | <0.001 |
|
|
|||||
| Drug | <0.001 | ||||
| RAS inhibitor | 437,158 (37.0)b,c,d | 35,540 (49.0)a,c,d | 87,838 (55.2)a,b,d | 16,857 (70.2)a,b,c | <0.001 |
| Sulfonylurea | 515,366 (43.6)b,c,d | 39,929 (55.0)a,c,d | 83,488 (52.5)a,b,d | 14,693 (61.2)a,b,c | <0.001 |
| Thiazolidinedione | 79,080 (6.7)b,c,d | 5,740 (7.9)a,d | 12,226 (7.7)a,d | 2,076 (8.7)a,b,c | <0.001 |
| DPP4 inhibitor | 96,743 (8.2)b,c,d | 7,027 (9.7)a,c | 13,468 (8.5)a,b,d | 2,344 (9.8)a,c | <0.001 |
| α-Glucosidase inhibitor | 137,279 (11.6)b,c,d | 12,468 (17.2)a,d | 26,780 (16.8)a,d | 5,856 (24.4)a,b,c | <0.001 |
| Meglitinide | 18,226 (1.5)b,c,d | 1,563 (2.2)a,c,d | 4,470 (2.8)a,b,d | 1,234 (5.1)a,b,c | <0.001 |
| Metformin | 560,669 (47.5)b,c,d | 41,886 (57.7)a,c,d | 83,506 (52.5)a,b,d | 13,720 (57.1)a,b,c | <0.001 |
|
|
|||||
| Systolic blood pressure, mm Hg | 129.6±15.8b,c,d | 134.2±17.6a,c,d | 130.5±16.4a,b,d | 135.4±18.6a,b,c | <0.001 |
|
|
|||||
| Diastolic blood pressure, mm Hg | 79±10.1b,c,d | 81.2±11.1a,c,d | 78.2±10.3a,b,d | 79.6±11.3a,b,c | <0.001 |
|
|
|||||
| eGFR, mL/min/1.73 m2 | 88±32.7b,c,d | 86.5±32.6a,c,d | 48±15.1a,b,d | 44.8±14.0a,b,c | <0.001 |
|
|
|||||
| Non-HDL-C, mg/dL | 144.4±44.3b,d | 149.8±50.2a,c,d | 144.6±45.6b,d | 148.9±55.3a,b,c | <0.001 |
|
|
|||||
| Triglycerides, mg/dL | 139.8 (139.6–139.9)b,c,d | 156.1 (155.5–156.8)a,c,d | 146.1 (145.8–146.5)a,b,d | 160.7 (159.7–161.8)a,b,c | <0.001 |
|
|
|||||
| AST, IU/L | 25.9 (25.8–25.9)b,c,d | 27.7 (27.6–27.8)a,c,d | 25.0 (25.0–25.1)a,b,d | 24.3 (24.1–24.4)a,b,c | <0.001 |
|
|
|||||
| ALT, IU/L | 25.5 (25.5–25.6)b,c,d | 27.5 (27.3–27.6)a,c,d | 22.7 (22.6–22.7)a,b,d | 22.1 (21.9–22.2)a,b,c | <0.001 |
|
|
|||||
| GGT, IU/L | 35.1 (35.0–35.1)b,c,d | 43.8 (43.6–44.1)a,c,d | 29.8 (29.7–29.9)a,b,d | 32.4 (32.1–32.7)a,b,c | <0.001 |
DKD, diabetic kidney disease; PU−GFR−, no DKD; PU+GFR−, proteinuric DKD with normal eGFR; PU−GFR+, non-proteinuric DKD with reduced eGFR; PU+GFR+, proteinuric DKD with reduced eGFR; BMI, body mass index; OHA, oral hypoglycemic agent; DR, diabetic retinopathy; RAS, renin-angiotensin system; DPP4, dipeptidyl peptidase 4; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase.
Table 2
Values are expressed as number (%) or hazard ratio (95% confidence interval). Model 1: unadjusted; Model 2: adjusted for age and sex; Model 3: Model 2+smoking, alcohol, exercise, and income; Model 4: Model 3+hypertension, dyslipidemia, and body mass index; Model 5: Model 4+stroke, proliferative diabetic retinopathy, insulin use, sulfonylurea use, thiazolidinedione use, renin-angiotensin system inhibitor use, and duration of diabetes (≥5 or <5 years).
Table 3
Values are expressed as number (%) or hazard ratio (95% confidence interval). Model 1: unadjusted; Model 2: adjusted for age and sex; Model 3: Model 2+smoking, alcohol, exercise, and income; Model 4: Model 3+hypertension, dyslipidemia, and body mass index; Model 5: Model 4+stroke, proliferative diabetic retinopathy, insulin use, sulfonylurea use, thiazolidinedione use, renin-angiotensin system inhibitor use, and duration of diabetes (≥5 or <5 years).




 Citation
Citation Print
Print



 XML Download
XML Download