Abstract
Background
The association between Graves’ disease (GD) and co-existing thyroid cancer is still controversial and most of the previously reported data have been based on surgically treated GD patients. This study investigated the clinicopathological findings and prognosis of concomitant thyroid cancer in GD patients in the era of widespread application of ultrasonography.
Methods
Data of GD patients who underwent thyroidectomy for thyroid cancer between 2010 and 2019 in three tertiary hospitals in South Korea (Asan Medical Center, Chonnam National University Hwasun Hospital, and Pusan National University Hospital) were collected and analyzed retrospectively. In the subgroup analysis, aggressiveness and clinical outcomes of thyroid cancer were compared nodular GD and non-nodular GD groups according to the presence or absence of the thyroid nodules other than thyroid cancer (index nodules).
Results
Of the 15,159 GD patients treated at the hospitals during the study period, 262 (1.7%) underwent thyroidectomy for coexisting thyroid cancer. Eleven patients (4.2%) were diagnosed with occult thyroid cancer and 182 patients (69.5%) had microcarcinomas. No differences in thyroid cancer aggressiveness, ultrasonographic findings, or prognosis were observed between the nodular GD and non-nodular GD groups except the cancer subtype. In the multivariate analysis, only lymph node (LN) metastasis was an independent prognostic factor for recurrent/persistent disease of thyroid cancer arising in GD (P=0.020).
Conclusion
The prevalence of concomitant thyroid cancer in GD patients was considerably lower than in previous reports. The clinical outcomes of thyroid cancer in GD patients were also excellent but, more cautious follow-up is necessary for patients with LN metastasis in the same way as for thyroid cancer in non-GD patients.
Graves’ disease (GD) is an autoimmune thyroid disease (AITD) that most frequently causes hyperthyroidism [1]. Binding to the G-protein couple thyrotropin receptor of circulating anti-thyroid-stimulating hormone (TSH) receptor antibodies results in thyrotoxicosis due to excessive thyroid hormone synthesis and thyroid gland hypertrophy [2,3]. The prevalence of thyroid cancer in GD patients varies widely, but a recent meta-analysis found a roughly 2.5-fold higher rate of thyroid cancer in GD patients than in the general population [4]. Early findings suggested that GD has a protective effect against thyroid cancer [5–7], but recent studies have revealed a higher incidence of thyroid cancer in patients with GD, particularly surgically treated patients [8,9]. Two nationwide cohort studies in Taiwan and Sweden evaluated the association between thyroid cancer and GD, and both found a higher incidence of thyroid cancer during the early GD follow-up period [10,11]. Routinely performed ultrasonography (US) in GD patients during disease surveillance might be related to overdiagnosis of thyroid cancer. Use of screening US for thyroid disease increases the probability of detecting thyroid nodules and patients with thyroid nodules may be at an increased risk for thyroid cancer [12]. A review and meta-analysis of surgically treated GD patients revealed that thyroid nodules are a risk factor of thyroid cancer [13], but the real incidence of thyroid cancer in GD patients who are treated with or without surgery is uncertain.
Studies investigating the clinicopathological findings and prognosis of thyroid cancer in GD patients have reported inconsistent data. Some have reported more aggressiveness [14] and higher recurrence risk in GD patients compared with euthyroid patients [15,16]. However, others have reported no differences in the clinical characteristics or outcomes of thyroid cancer arising in GD patients [17,18], and one study reported a better prognosis of thyroid cancer in GD patients [19]. These inconsistencies may be related to the small sample sizes and variety of clinical settings in previous studies. Therefore, the present retrospective multicenter study was conducted to evaluate the clinicopathological features and outcomes of thyroid cancer in GD patients in the era of widespread application of US.
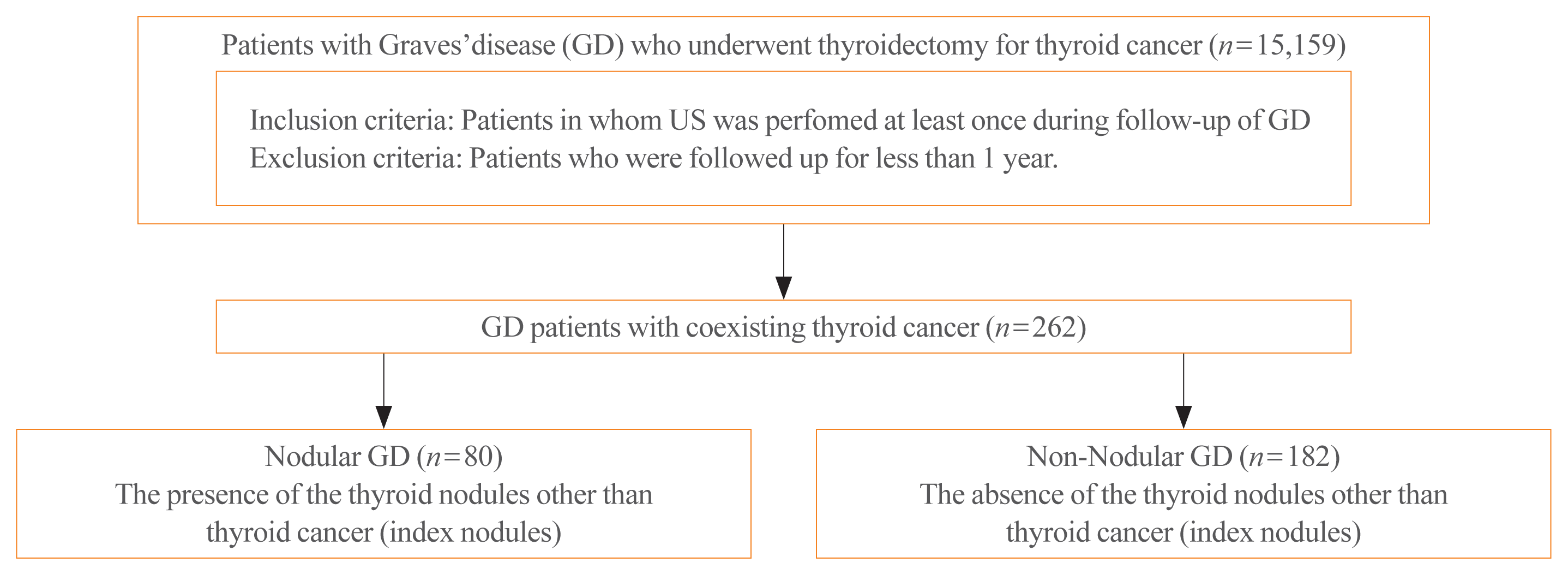
This retrospective multicenter study was conducted at three tertiary referral hospitals in South Korea: Asan Medical Center, Chonnam National University Hwasun Hospital, and Pusan National University Hospital. Data were collected for all GD patients who underwent thyroid cancer surgery between January 2010 and December 2019. The GD diagnostic criteria were defined as biochemical evidence of hyperthyroidism with serum anti-thyrotropin receptor antibody (thyrotropin binding inhibiting immunoglobulin [TBII]), and/or increased diffuse 123I or 99mTc-pertechnetate uptake on a radionuclide scan, and/or the presence of clinical features, such as goiter or Graves’ ophthalmopathy [20]. Patients in whom US was performed at least once during follow-up of GD were included. Thyroid US was performed using iU22 unit (Philips Healthcare, Bothell, WA, USA), EUB-7500 unit (Hitachi Medical Systems, Tokyo, Japan), GE Logiq E8 and E9 (Milwaukee-Brookfield, WI, USA), or Siemens ACUSON S2000 (Malvern, PA, USA) with a linear high-frequency probe (5 to 14 MHz). Patients who were followed up for less than 1 year were excluded.
The final study population consisted of 262 GD patients with coexisting thyroid cancer among 15,159 GD patients during the study period. The study protocol was approved by the Institutional Review Board (IRB) of each participating institute and the need for informed consent was waived due to the retrospective design (IRB approval numbers; IRB 2020-1776, CNUHH-2021-136, 2011-008-096).
Demographic data including age, sex, smoking status, and prevalence of comorbidities, including hypertension, diabetes mellitus, dyslipidemia, and cardiovascular disease were collected from medical records. A family history of GD, GD activity status, type of anti-thyroid medication, and the TBII titer at the time of GD diagnosis were investigated retrospectively. Active GD status was defined as newly diagnosed GD or taking anti-thyroid drugs. The reference range of TBII was 0 to 1.5 IU/L. GD patients were divided into two groups according to the presence or absence of the thyroid nodules other than thyroid cancer (index nodules): nodular GD and non-nodular GD groups (Fig. 1).
Medical records yielded clinicopathological findings including coexisting thyroid cancer, tumor size, histological type, presence of extra-thyroidal extension (ETE), presence of cervical lymph node (LN) metastasis, number and longest diameter of metastatic LN, presence of distant metastasis, and initial tumor-node-metastasis (TNM) stage according to the 8th edition of the American Joint Committee on Cancer/Union for International Cancer Control TNM staging system [21], presence of the thyroid nodules other than thyroid cancer (index nodules), thyroid volume (g), extent of surgery, and use of radioactive iodine (RAI) therapy with total dose (mCi).
Serum TSH, free thyroxine, anti-thyroglobulin (Tg) antibody, and Tg levels were measured every 6 to 12 months during follow-up. Regular US follow-up was also performed every 6 to 12 months, and fine-needle aspiration (FNA) and/or washout fluid Tg measurements were conducted to detect recurrence if suspicious lesions were observed. Clinical outcomes of thyroid cancer were divided into remission, recurrence, persistence of biochemical disease, and persistence of structural disease as follows; remission, no clinical, biochemical, or structural evidence of disease; recurrence, evidence of disease after at least 1 year of remission; persistence of biochemical disease, persistently abnormal suppressed and/or stimulated Tg values or elevated anti-Tg antibody titer without structural evidence of disease; and persistence of structural disease, structural or functional (RAI scan, 18F-fluorodeoxyglucose positron emission tomography [18FDG-PET]) evidence of loco-regional or distant metastases.
Data are expressed as mean±standard deviation, median (interquartile range), or number (%). Continuous variables were analyzed using Student’s t test, and categorical variables were analyzed using the chi-square test. Binary logistic regression analyses were used to assess the risk factors for recurrence/persistence of thyroid cancer. Continuous variable (TBII titer at diagnosis) with missing data were replaced with the mean value. All statistical analyses were performed using SPSS Statistics version 25 software (IBM Corp., Armonk, NY, USA), and a P value <0.05 was considered significant.
Of the 15,159 GD patients treated during the study period, 262 (1.7%) were diagnosed with thyroid cancer. Most patients underwent thyroid surgery due to cytologically suspicious or a proven malignancy (n=251, 95.8%) or other reasons, such as compressive symptoms due to a large goiter or relapsed and/or uncontrolled hyperthyroidism (n=11, 4.2%). Among the 262 patients, 245 (93.5%) underwent total thyroidectomy and 17 (6.5%) underwent lobectomy. Table 1 lists the baseline characteristics of these patients. The mean age was 55.1±13.9 years, and 78.6% were female. Active GD status was observed in 87.4% (n=229) of the 262 patients; 200 patients had newly diagnosed GD, and 29 patients had recurrent GD. The mean TBII titer at the time of the first GD diagnosis was 16.0±19.6 IU/L. Of the 262 patients, 11 (4.2%) were diagnosed with occult thyroid cancer after total thyroidectomy for other reasons and 182 patients (69.5%) had microcarcinoma. During the study period, 19,397 (64.2%) of 30,213 non-GD patients underwent thyroid cancer surgery in three tertiary hospitals revealed to have microcarcinomas, which is comparable to GD patients.
The clinicopathological findings of GD patients with coexisting thyroid cancer were compared between the nodular GD and non-nodular GD groups (Table 2). The nodular GD group was older than the non-nodular group (61.9±12.8 years vs. 52.1±13.3 years, P<0.001) and showed a female-dominance (86.3% vs. 75.3%, P=0.046). The GD activity and preoperative FNA results did not differ between the two groups. All patients in the non-nodular GD group had papillary thyroid carcinoma (PTC); the nodular GD group included two cases of non-PTC including one case of follicular carcinoma (FTC) and one case of poorly differentiated thyroid carcinoma (PDTC) (P=0.043). Subgroup analysis of PTC revealed that more follicular variant PTC was observed in the nodular GD group than the non-nodular group. Seven cases subtypes of PTC other than follicular variant PTC were found only in the non-nodular GD group: three cases of tall cell variant PTC, one case of oncocytic variant PTC, two cases of solid variant PTC, and one case of diffuse sclerosing variant PTC (Table 3). Mean thyroid volume (g) was higher in the nodular GD group than in the non-nodular GD group (42.9±35.5 g vs. 33.1±30.4 g, P=0.036). No significant differences in tumor size, bilaterality, lymphovascular invasion, ETE, or LN metastasis were observed between the two groups. Distant metastasis was not observed. RAI therapy was performed at similar rates in both groups (42.5% vs. 43.4%, P=0.891). The clinical outcomes of thyroid cancer were similar between the two groups during the follow-up period (mean 59.1 months). At the last follow-up, 242 patients (92.4%) were in thyroid cancer remission status. Recurrence was observed in eight patients (4.4%) in the non-nodular GD group. Additionally, preoperative US findings of thyroid cancer did not differ between the nodular GD and non-nodular GD groups (Supplemental Table S1).
The univariate analysis revealed that tumor size (odds ratio [OR], 2.463; P=0.001), ETE (OR, 3.400; P=0.010), LN metastasis (OR, 9.260; P<0.001), and RAI therapy (OR, 8.618; P=0. 001) were significantly associated with the risk of recurrence/persistent disease. However, the multivariate analysis, only LN metastasis (OR, 4.359; P=0.020) was independently associated with recurrence/persistent disease (Table 4).
Unlike most previous studies investigating the prevalence of thyroid cancer in GD patients who underwent thyroidectomy, the present study enrolled all GD patients with thyroid cancer who were treated at three tertiary hospitals. Data from previous studies regarding the prevalence of coexisting thyroid cancer in GD patients assessed preoperatively by US are summarized in Supplemental Table S2 [9,16,22–30]. Studies using US to find thyroid nodules reported varying rates of concomitant thyroid cancer in GD patients were reported (3.3% to 28.2%). The reported prevalence rates of coexisting thyroid cancers in GD patients have historically been lower, so some authors suggested that GD may have a protective effect against thyroid cancer, such as an anti-tumor effect via GD autoimmunity [6,31]; however, most thyroid cancers were detected as incidentalomas postoperatively or as palpable nodules preoperatively in the pre-US era, which may have led the underestimation of thyroid cancer. To avoid selection and surveillance bias, many studies have compared the prevalence of occult papillary thyroid microcarcinoma (PTMC) in GD patients to those with multi-nodular goiters or chronic lymphocytic thyroiditis undergoing thyroidectomy for noncancer-related reasons [32,33]. These studies also yielded inconsistent findings due to their small sample sizes. Recent studies in the era of widespread adoption of US showed a higher prevalence rate of thyroid cancer in GD patients compared to earlier studies. The immune tolerance of AITD prevents thyroid failure, which may increase the risk for thyroid cancer in GD patients [34,35] and binding of thyroid-stimulating antibody (TSAb) to the thyrotropin receptor stimulates tumor formation and angiogenesis by upregulating various growth factors [8,36].
In the present study, thyroid cancer was detected in only 1.7% of all GD patients during the study period, which is much lower than in most previous reports [4]. However, a Japanese study reported only 166 cytologically diagnosed thyroid cancers among 32,200 GD patients who were treated and underwent thyroid US between 1994 and 2004 in an outpatient clinic [22], which was an even lower incidence of thyroid cancer than in our study. A prospective Korean study reported a 3.3% prevalence of thyroid cancer in 245 GD patients after screening US was performed in all patients [23]. In only a few studies, US was performed in all GD patients, including non-surgically treated patients as in our study, and these studies reported lower incidences of thyroid cancer. A recent multicenter study performing preoperative US in all GD patients reported that only 38.3% of GD patients with coexisting thyroid cancer underwent thyroidectomy with suspicious FNA results and the ratio of microcarcinoma (<1 cm) was significantly higher in GD patients compared to age, sex, and tumor size-matched non-GD patients (60% vs. 37%) [37]. Our study revealed a similar ratio of microcarcinoma in GD patients, even though thyroid cancer was diagnosed by preoperative US-guided FNA in 95.8% of GD patients. The increased ratio of PTMC in GD patients in recent studies could be related to the use of high-resolution US, which has been linked to an increase in thyroid cancer incidence in the last two decades [38]. In particular, a remarkable rise in thyroid cancer incidence has been observed in Korea [39].
A previous review and meta-analysis found that the presence of thyroid nodules increases the risk of thyroid cancer [4,13]. Up to half of GD patients with coexisting thyroid cancer have a nodular goiter on preoperative US [9], and we also found that 80 of the 262 GD patients had other thyroid nodule other than thyroid cancer. Our study analyzed clinical characteristics and prognosis of thyroid cancer arising from GD according to the presence of multi-nodularity to determine the association between thyroid autoimmune nodular changes and thyroid cancer. Nodular GD was more frequently observed in older women, but we found no difference in thyroid cancer characteristics at the time of the thyroid cancer diagnosis except for the histological type. FTC and PDTC were observed in only nodular GD patients, but almost all GD patients had PTC. All cases of the aggressive subtypes of PTC, such as the tall cell variant and solid variant, were observed in non-nodular GD patients. A study conducted in Greece [40] found a higher incidence of tall cell variant PTC in GD patients than non-GD patients, but we found a relatively low incidence of tall cell variant PTC in GD patients, which could be due to ethnic differences. Our subgroup analysis of US findings for thyroid cancer revealed no differences according to the presence of other thyroid nodules. It is important to screen for well-known suspicious US features [41] when attempting to detect thyroid cancer.
Previous studies investigating the link between GD and the prognosis of coexisting thyroid cancer have reported inconsistent data. One study found that in patients with thyroid cancers <1 cm, GD patients had an excellent prognosis and longer disease-free survival compared to age, sex, and tumor size-matched non-GD patients [19]. However, a meta-analysis found increased multifocality and distant metastasis at the time of the differentiated thyroid carcinoma (DTC) diagnosis in GD patients compared to non-GD patients [42]. A small study with a relatively long follow-up period (median 165.6 months) observed that GD patients with coexisting DTC had a poorer prognosis compared with age, sex, and tumor size-matched non-GD patients [43]. The same study found that 33.3% (7/21) of GD patients and 10.0% (7/70) of non-GD patients were classified as stage IV on the TNM 7th edition [43]. It reported that six of the GD patients died, including three patients with distant metastasis at the time of the thyroid cancer diagnosis, whereas only one patient died in the non-GD group; However, it observed recurrent/persistent disease in 14.3% of the GD patients with DTC with only biochemical evidence, but in 10.0% of non-GD patients with structural evidence including LN and distant metastases [43]. In the present study, we did not detect any distant metastasis in any of the GD patients with DTC, which may have been due to early detection of thyroid cancer at the time of the GD diagnosis. We observed recurrence of thyroid cancer only in non-nodular GD patients, although the difference was not significant. GD may not harm the prognosis of non-aggressive thyroid cancer, although this finding is based on nodular GD reported as chronic stimulation of TSAbs. Only LN metastasis was an independent prognostic factor for recurrence/persistent disease of thyroid cancer in GD patients. Advanced thyroid cancer with metastasis or a more aggressive histological type could be stimulated by the pathomechanism of GD, so early metastatic spread and increased mortality may occur.
This study had several limitations due to its retrospective design. We could not directly compare the prevalence of thyroid cancer between GD patients and non-GD patients. Additionally, the association between TBII in thyroid cancer and GD patients was evaluated using only the TBII titer at the time of the GD diagnosis. We could not evaluate the association with serial follow-up TBII and thyroid-stimulating immunoglobulin on thyroid cancer due to a lack of data. The follow-up period for thyroid cancer was also relatively short to evaluate the long-term clinical outcome. Large-scale prospective studies are necessary to accurately investigate the association between thyroid cancer and GD.
In conclusion, we found that the prevalence of thyroid cancer was much lower than in previous reports of GD patients. Additionally, more than 60% of the thyroid cancers arising from GD were microcarcinomas even though most of the thyroid cancers were diagnosed preoperatively by US-guided FNA. The clinical outcomes of thyroid cancer in GD patients were also excellent but, closer follow-up is necessary for patients with LN metastasis in the same way as for thyroid cancer in non-GD patients.
Supplementary Information
Supplemental Table S1.
Subgroup Analysis for Ultrasonographic Features between Nodular GD and Non-Nodular GD (n=251)
Supplemental Table S2.
Clinical Characteristics of Coexisting Thyroid Cancer in GD Patients Who Were Performed Ultrasonography Reported in Literature
REFERENCES
2. Wemeau JL, Klein M, Sadoul JL, Briet C, Velayoudom-Cephise FL. Graves’ disease: introduction, epidemiology, endogenous and environmental pathogenic factors. Ann Endocrinol (Paris). 2018; 79:599–607.

3. Bartalena L. Diagnosis and management of Graves disease: a global overview. Nat Rev Endocrinol. 2013; 9:724–34.

4. Staniforth JU, Erdirimanne S, Eslick GD. Thyroid carcinoma in Graves’ disease: a meta-analysis. Int J Surg. 2016; 27:118–25.

5. Gabriele R, Letizia C, Borghese M, De Toma G, Celi M, Izzo L, et al. Thyroid cancer in patients with hyperthyroidism. Horm Res. 2003; 60:79–83.

6. Sokal JE. Incidence of malignancy in toxic and nontoxic nodular goiter. J Am Med Assoc. 1954; 154:1321–5.

7. Rieger R, Pimpl W, Money S, Rettenbacher L, Galvan G. Hyperthyroidism and concurrent thyroid malignancies. Surgery. 1989; 106:6–10.
8. Pazaitou-Panayiotou K, Michalakis K, Paschke R. Thyroid cancer in patients with hyperthyroidism. Horm Metab Res. 2012; 44:255–62.

9. Keskin C, Sahin M, Hasanov R, Aydogan BI, Demir O, Emral R, et al. Frequency of thyroid nodules and thyroid cancer in thyroidectomized patients with Graves’ disease. Arch Med Sci. 2019; 16:302–7.

10. Shu X, Ji J, Li X, Sundquist J, Sundquist K, Hemminki K. Cancer risk in patients hospitalised for Graves’ disease: a population-based cohort study in Sweden. Br J Cancer. 2010; 102:1397–9.

11. Chen YK, Lin CL, Chang YJ, Cheng FT, Peng CL, Sung FC, et al. Cancer risk in patients with Graves’ disease: a nationwide cohort study. Thyroid. 2013; 23:879–84.

12. Belfiore A, Russo D, Vigneri R, Filetti S. Graves’ disease, thyroid nodules and thyroid cancer. Clin Endocrinol (Oxf). 2001; 55:711–8.

13. Papanastasiou A, Sapalidis K, Goulis DG, Michalopoulos N, Mareti E, Mantalovas S, et al. Thyroid nodules as a risk factor for thyroid cancer in patients with Graves’ disease: a systematic review and meta-analysis of observational studies in surgically treated patients. Clin Endocrinol (Oxf). 2019; 91:571–7.

14. Ozaki O, Ito K, Kobayashi K, Toshima K, Iwasaki H, Yashiro T. Thyroid carcinoma in Graves’ disease. World J Surg. 1990; 14:437–41.

15. Menon R, Nair CG, Babu M, Jacob P, Krishna GP. The outcome of papillary thyroid cancer associated with Graves’ disease: a case control study. J Thyroid Res. 2018; 2018:8253094.

16. Cappelli C, Braga M, De Martino E, Castellano M, Gandossi E, Agosti B, et al. Outcome of patients surgically treated for various forms of hyperthyroidism with differentiated thyroid cancer: experience at an endocrine center in Italy. Surg Today. 2006; 36:125–30.

17. Kasuga Y, Sugenoya A, Kobayashi S, Masuda H, Iida F. The outcome of patients with thyroid carcinoma and Graves’ disease. Surg Today. 1993; 23:9–12.

18. Hales IB, McElduff A, Crummer P, Clifton-Bligh P, Delbridge L, Hoschl R, et al. Does Graves’ disease or thyrotoxicosis affect the prognosis of thyroid cancer. J Clin Endocrinol Metab. 1992; 75:886–9.

19. Kikuchi S, Noguchi S, Yamashita H, Uchino S, Kawamoto H. Prognosis of small thyroid cancer in patients with Graves’ disease. Br J Surg. 2006; 93:434–9.

20. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016; 26:1343–421.

21. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. 2017 AJCC Cancer Staging Manual. 8th ed. New York: Springer;2017.
22. Yano Y, Shibuya H, Kitagawa W, Nagahama M, Sugino K, Ito K, et al. Recent outcome of Graves’ disease patients with papillary thyroid cancer. Eur J Endocrinol. 2007; 157:325–9.

23. Kim WB, Han SM, Kim TY, Nam-Goong IS, Gong G, Lee HK, et al. Ultrasonographic screening for detection of thyroid cancer in patients with Graves’ disease. Clin Endocrinol (Oxf). 2004; 60:719–25.

24. Kraimps JL, Bouin-Pineau MH, Mathonnet M, De Calan L, Ronceray J, Visset J, et al. Multicentre study of thyroid nodules in patients with Graves’ disease. Br J Surg. 2000; 87:1111–3.

25. Erbil Y, Barbaros U, Ozbey N, Kapran Y, Tukenmez M, Bozbora A, et al. Graves’ disease, with and without nodules, and the risk of thyroid carcinoma. J Laryngol Otol. 2008; 122:291–5.

26. Ren M, Wu MC, Shang CZ, Wang XY, Zhang JL, Cheng H, et al. Predictive factors of thyroid cancer in patients with Graves’ disease. World J Surg. 2014; 38:80–7.

27. Ergin AB, Saralaya S, Olansky L. Incidental papillary thyroid carcinoma: clinical characteristics and prognostic factors among patients with Graves’ disease and euthyroid goiter, Cleveland Clinic experience. Am J Otolaryngol. 2014; 35:784–90.

28. Tam AA, Kaya C, Kilic FB, Ersoy R, Cakir B. Thyroid nodules and thyroid cancer in Graves’ disease. Arq Bras Endocrinol Metabol. 2014; 58:933–8.

29. Alvarez AL, Mulder M, Handelsman RS, Lew JI, Farra JC. High rates of underlying thyroid cancer in patients undergoing thyroidectomy for hyperthyroidism. J Surg Res. 2020; 245:523–8.

30. Casella C, Morandi R, Verrengia A, Galani A, Molfino S, Cuka D, et al. Thyroid cancer and nodules in Graves’ disease: a single center experience. Endocr Metab Immune Disord Drug Targets. 2021; 21:2028–34.

31. Imam S, Dar P, Paparodis R, Almotah K, Al-Khudhair A, Hasan SA, et al. Nature of coexisting thyroid autoimmune disease determines success or failure of tumor immunity in thyroid cancer. J Immunother Cancer. 2019; 7:3.

32. Paparodis RD, Karvounis E, Bantouna D, Chourpiliadis C, Chourpiliadi H, Livadas S, et al. Incidentally discovered papillary thyroid microcarcinomas are more frequently found in patients with chronic lymphocytic thyroiditis than with multinodular goiter or Graves’ disease. Thyroid. 2020; 30:531–5.

33. Jia Q, Li X, Liu Y, Li L, Kwong JS, Ren K, et al. Incidental thyroid carcinoma in surgery-treated hyperthyroid patients with Graves’ disease: a systematic review and meta-analysis of cohort studies. Cancer Manag Res. 2018; 10:1201–7.
34. Lee HJ, Li CW, Hammerstad SS, Stefan M, Tomer Y. Immunogenetics of autoimmune thyroid diseases: a comprehensive review. J Autoimmun. 2015; 64:82–90.

35. Ferrari SM, Fallahi P, Elia G, Ragusa F, Ruffilli I, Paparo SR, et al. Thyroid autoimmune disorders and cancer. Semin Cancer Biol. 2020; 64:135–46.

36. Filetti S, Belfiore A, Amir SM, Daniels GH, Ippolito O, Vigneri R, et al. The role of thyroid-stimulating antibodies of Graves’ disease in differentiated thyroid cancer. N Engl J Med. 1988; 318:753–9.

37. Premoli P, Tanda ML, Piantanida E, Veronesi G, Gallo D, Masiello E, et al. Features and outcome of differentiated thyroid carcinoma associated with Graves’ disease: results of a large, retrospective, multicenter study. J Endocrinol Invest. 2020; 43:109–16.

38. Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic?: the increasing impact of overdiagnosis. N Engl J Med. 2016; 375:614–7.

39. Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid. 2015; 25:1127–36.

40. Boutzios G, Vasileiadis I, Zapanti E, Charitoudis G, Karakostas E, Ieromonachou P, et al. Higher incidence of tall cell variant of papillary thyroid carcinoma in Graves’ disease. Thyroid. 2014; 24:347–54.

41. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.

Table 1
Baseline Characteristics of Thyroid Cancer Patients with Graves’ Disease (n=262)
Table 2
Clinicopathological Findings of Coexisting Thyroid Cancer between Nodular GD and Non-Nodular Graves’ Disease (n=262)
| Variable | Total | Nodular GD (n=80) | Non-nodular GD (n=182) | P value |
|---|---|---|---|---|
| Age, yr | 55.1±13.9 | 61.9±12.8 | 52.1±13.3 | <0.001 |
|
|
||||
| Female sex | 206 (78.6) | 69 (86.3) | 137 (75.3) | 0.046 |
|
|
||||
| Active GD | 229 (87.4) | 70 (87.5) | 159 (87.4) | 0.975 |
|
|
||||
| Preoperative FNA resultsa | 251 | 74 | 177 | 0.351 |
| Non-diagnostic | 18 (7.2) | 6 (8.1) | 12 (6.6) | |
| Benign | 3 (1.2) | 1 (1.4) | 2 (1.2) | |
| AUS/FLUS | 7 (2.8) | 3 (4.1) | 4 (2.4) | |
| Follicular neoplasm | 2 (0.8) | 1 (1.4) | 1 (0.6) | |
| Suspicious malignancy | 60 (23.9) | 18 (24.3) | 42 (23.7) | |
| Malignancy | 161 (64.1) | 45 (60.8) | 116 (65.5) | |
|
|
||||
| Cancer type | 0.043 | |||
| PTC | 260 (99.2) | 78 (97.5) | 182 (100) | |
| FTC | 1 (0.4) | 1 (1.3) | 0 | |
| PDTC | 1 (0.4) | 1 (1.3) | 0 | |
|
|
||||
| Type of operation | 0.233 | |||
| Lobectomy | 17 (6.5) | 3 (3.8) | 14 (7.7) | |
| Total thyroidectomy | 245 (93.5) | 77 (96.3) | 168 (92.3) | |
|
|
||||
| Pathological findings | ||||
| Tumor size, cm | 0.1±0.6 | 0.9±0.7 | 1.0±0.6 | 0.358 |
| Bilaterality | 54 (20.6) | 20 (25.0) | 34 (18.7) | 0.244 |
| Lymphovascular invasion | 26 (10.0) | 7 (8.8) | 19 (10.5) | 0.664 |
| ETE | 0.653 | |||
| No | 197 (75.2) | 59 (73.8) | 138 (75.8) | |
| Micro | 56 (21.4) | 17 (21.3) | 39 (21.4) | |
| Macro | 9 (3.4) | 4 (5.0) | 5 (2.7) | |
| LN metastasis | 0.489 | |||
| No | 173 (66.0) | 56 (70.0) | 117 (64.3) | |
| N1a | 73 (27.9) | 21 (26.3) | 52 (28.6) | |
| N1b | 16 (6.1) | 3 (3.8) | 13 (7.1) | |
| Number of involved LNs | 1.8±6.0 | 1.7±7.8 | 1.9±5.0 | 0.858 |
| The largest diameter, cm | 0.5±0.6 | 0.8±0.8 | 0.5±0.6 | 0.151 |
| Thyroid gland volume, gb | 36.1±32.3 | 42.9±35.5 | 33.1±30.4 | 0.036 |
|
|
||||
| RAI therapy | 113 (43.1) | 34 (42.5) | 79 (43.4) | 0.891 |
| Total dose of 131I, mCi | 99.6±75.7 | 98.9±54.5 | 99.9±83.8 | 0.945 |
|
|
||||
| Clinical outcome | 0.840 | |||
| Remission | 242 (92.4) | 76 (95.0) | 166 (91.2) | |
| Recurrence | 8 (3.1) | 0 | 8 (4.4) | |
| Persistent, structural | 9 (3.4) | 2 (2.5) | 7 (3.8) | |
| Persistent, biochemical | 3 (1.1) | 2 (2.5) | 1 (0.5) | |
|
|
||||
| Mean follow-up, mo | 59.1±37.5 | 57.8±36.7 | 59.7±37.9 | 0.713 |
GD, Graves’ disease; FNA, fine needle aspiration; AUS, atypia of undetermined significance; FLUS, follicular lesion of undetermined significance; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma; PDTC, poorly differentiated thyroid carcinoma; ETE, extrathyroidal extension; LN, lymph node; RAI, radioactive iodine.
Table 3
Subgroup Analysis of Cancer Subtype in Graves’ Disease Patients with Coexisting Papillary Thyroid Cancer (n=260)
Table 4
The Risk Factors for Recurrent/Persistent Disease of Thyroid Cancer in Patients with Graves’ Disease




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download