Abstract
Background
Methods
Results
Supplementary Information
Supplemental Table S1.
Supplemental Table S2.
Supplemental Fig. S1.
Supplemental Fig. S2.
ACKNOWLEDGMENTS
REFERENCES
Fig. 1
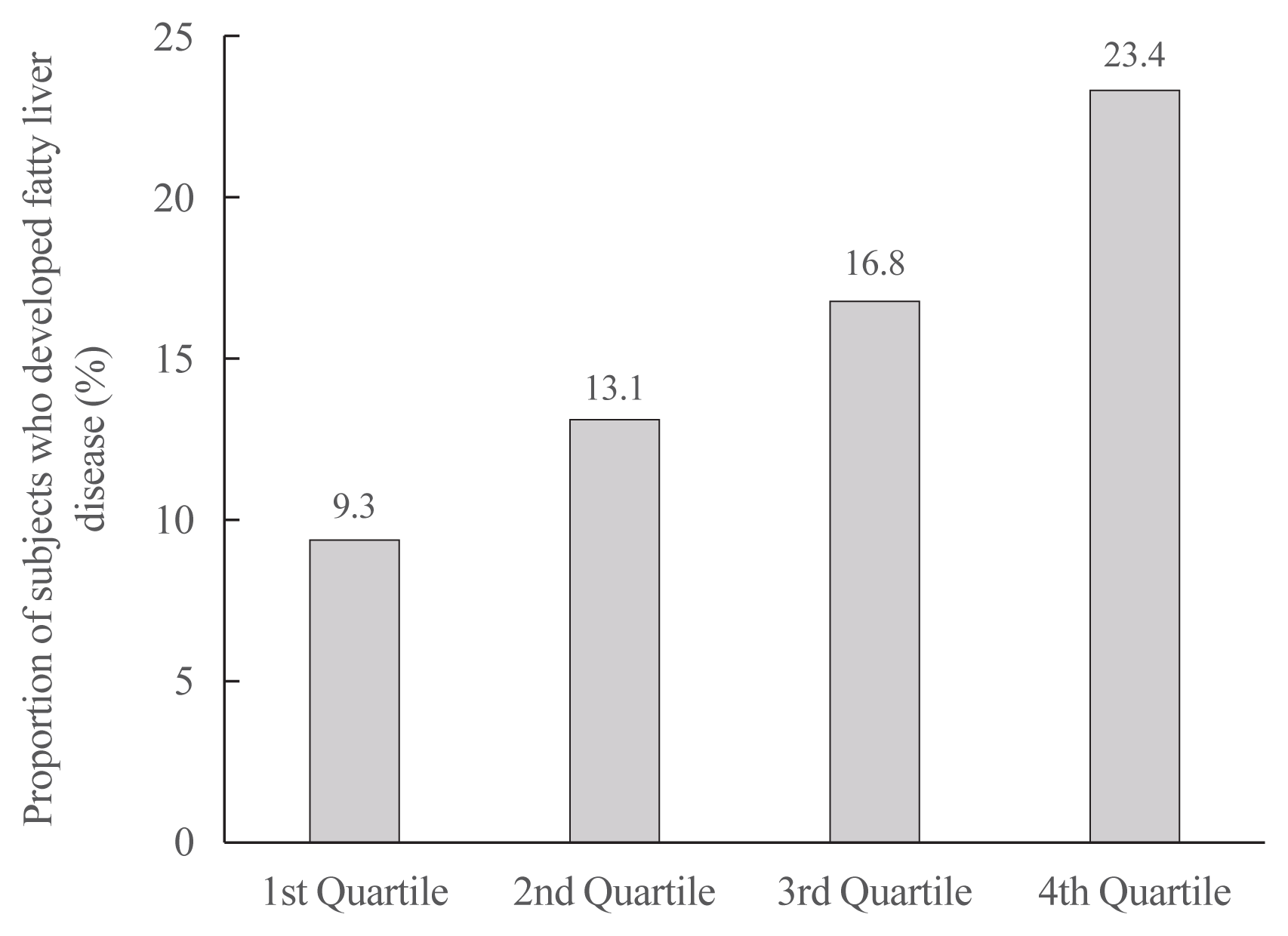
Fig. 2
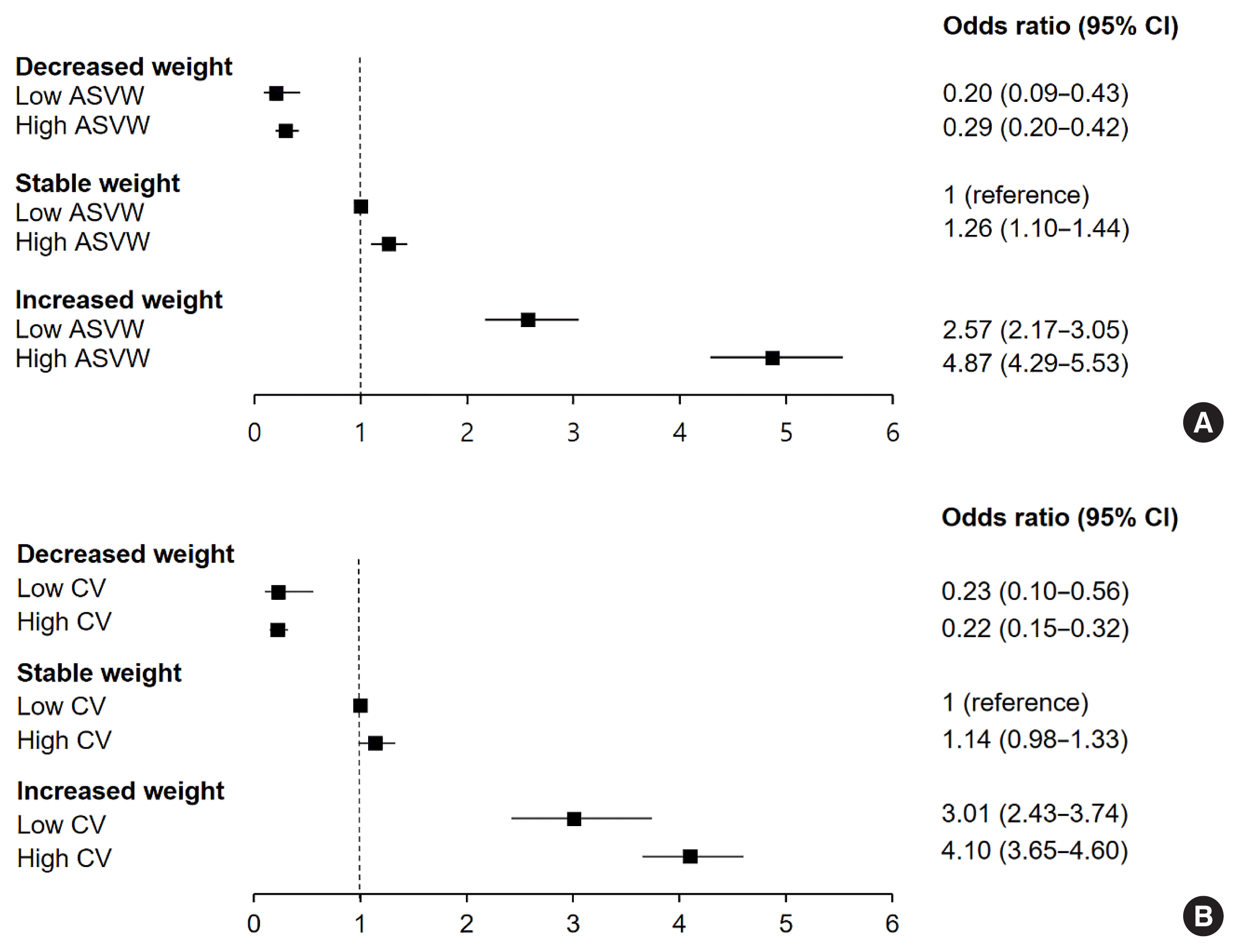
Table 1
| Characteristic | Total (n=15,340) | Presence of fatty liver disease after 4 years | P value | |
|---|---|---|---|---|
| No (n=12,934) | Yes (n=2,406) | |||
| Age, yr | 38.1±5.8 | 38.1±5.7 | 38.4±6.0 | 0.003 |
| Male sex | 6,514 (42.5) | 4,833 (37.4) | 1,681 (69.9) | <0.001 |
| BMI, kg/m2 | 21.9±2.6 | 21.5±2.5 | 23.8±2.5 | <0.001 |
| Waist circumference, cm | 77.7±7.8 | 76.6±7.4 | 83.5±7.0 | <0.001 |
| HOMA-IRa | 1.1±0.6 | 1.1±0.6 | 1.3±0.7 | <0.001 |
| HbA1c, % | 5.6±0.2 | 5.5±0.2 | 5.6±0.2 | <0.001 |
| Fasting blood glucose, mg/dL | 92.1±7.8 | 91.7±7.8 | 94.1±7.7 | <0.001 |
| Total cholesterol, mg/dL | 188.0±31.4 | 186.5±31.1 | 196.3±31.9 | <0.001 |
| Triglyceride, mg/dL | 89.1±50.0 | 83.8±43.2 | 117.4±70.2 | <0.001 |
| HDL-C, mg/dL | 61.7±14.3 | 63.1±14.2 | 54.3±12.6 | <0.001 |
| LDL-C, mg/dL | 113.1±28.9 | 110.9±28.4 | 124.5±28.5 | <0.001 |
| AST, IU/La | 19.4±9.9 | 19.2±10.2 | 20.5±7.4 | <0.001 |
| ALT, IU/La | 17.4±10.7 | 16.6±10.2 | 21.6±12.2 | <0.001 |
| SBP, mm Hg | 105.6±12.1 | 104.7±11.9 | 110.3±11.8 | <0.001 |
| Alcohol intake, g/daya | 6.3±6.6 | 5.9±6.4 | 8.1±7.5 | <0.001 |
| Current smoker | 2,234 (14.6) | 1,569 (12.1) | 665 (27.6) | <0.001 |
| Regular exerciseb | 1,824 (11.9) | 1,548 (12.0) | 276 (11.5) | 0.912 |
| Obesityc | 1,784 (11.6) | 1,111 (8.6) | 673 (28.0) | 0.177 |
BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; AST, aspartate transaminase; ALT, alanine aminotransferase; SBP, systolic blood pressure.
a Right-skewed variables (HOMA-IR, AST, ALT, and alcohol intake) were log-transformed for the Student’s t tests;
Table 2
Values are expressed as odds ratio (95% confidence interval). Model 1: Adjusted for age and sex; Model 2: Adjusted for Model 1+fasting blood glucose, systolic blood pressure, triglyceride, low-density lipoprotein cholesterol, smoking status, exercise, and alcohol intake; Model 3: Adjusted for Model 2+homeostatic model assessment of insulin resistance, glycated hemoglobin, baseline body mass index, aspartate transaminase. Cut off value for ASVW: 1st quartile (<1.13 kg), 2nd quartile (1.13–1.625 kg), 3rd quartile (1.625–2.33 kg), 4th quartile (≥2.33 kg); Cut off value for CV: 1st quartile (<1.9 kg), 2nd quartile (1.9–2.71 kg), 3rd quartile (2.71–3.84 kg), 4th quartile (≥3.84 kg).
Table 3
| Presence of NAFLD | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| Normal and low ASVW | 655 (9.2) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Normal and high ASVW | 1,078 (16.7) | 1.84 (1.65–2.04) | 1.81 (1.63–2.02) | 1.80 (1.61–2.01) |
| Obese and low ASVWa | 203 (38.3) | 3.94 (3.23–4.80) | 3.12 (2.55–3.83) | 2.77 (2.26–3.41) |
| Obese and high ASVW | 470 (37.5) | 4.44 (3.84–5.13) | 3.52 (3.03–4.09) | 3.11 (2.66–3.62) |
| Normal and low CV | 673 (9.8) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Normal and high CV | 1,060 (15.8) | 1.99 (1.79–2.22) | 2.05 (1.84–2.29) | 2.05 (1.84–2.29) |
| Obese and low CVa | 301 (36.2) | 3.45 (2.92–4.07) | 2.80 (2.36–3.33) | 2.50 (2.10–2.97) |
| Obese and high CV | 372 (39.1) | 5.36 (4.57–6.28) | 4.27 (3.62–5.03) | 3.76 (3.18–4.44) |
Values are expressed as number (%) or odds ratio (95% confidence interval). Model 1: Adjusted for age and sex; Model 2: Adjusted for Model 1+fasting blood glucose, systolic blood pressure, triglyceride, low-density lipoprotein cholesterol, smoking status, exercise, and alcohol intake; Model 3: Adjusted for Model 2+homeostatic model assessment of insulin resistance, glycated hemoglobin, aspartate transaminase.
Table 4
| Presence of NAFLD | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| Non-obese to non-obese, low ASVW | 547 (8.1) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Non-obese to non-obese, high ASVW | 637 (11.7) | 1.42 (1.25–1.60) | 1.39 (1.23–1.58) | 1.39 (1.22–1.57) |
| Obese to obese, low ASVWa | 200 (41.8) | 5.21 (4.23–6.42) | 4.20 (3.39–5.21) | 3.76 (3.02–4.67) |
| Obese to obese, high ASVW | 457 (43.7) | 6.66 (5.70–7.78) | 5.43 (4.62–6.39) | 4.85 (4.11–5.72) |
| Non-obese to non-obese, low CV | 559 (8.6) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Non-obese to non-obese, high CV | 625 (10.9) | 1.54 (1.36–1.75) | 1.58 (1.39–1.79) | 1.58 (1.39–1.80) |
| Obese to obese, low CVa | 296 (39.1) | 4.50 (3.78–5.36) | 3.68 (3.07–4.41) | 3.30 (2.74–3.96) |
| Obese to obese, high CV | 361 (47.1) | 8.79 (7.4–10.46) | 7.32 (6.13–8.76) | 6.52 (5.44–7.82) |
Values are expressed as number (%) or odds ratio (95% confidence interval). Model 1: Adjusted for age and sex; Model 2: Adjusted for Model 1+fasting blood glucose, systolic blood pressure, triglyceride, low-density lipoprotein cholesterol, smoking status, exercise, and alcohol intake; Model 3: Adjusted for Model 2+homeostatic model assessment of insulin resistance, glycated hemoglobin, aspartate transaminase.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download