1. Russ G, Leboulleux S, Leenhardt L, Hegedus L. Thyroid incidentalomas: epidemiology, risk stratification with ultrasound and workup. Eur Thyroid J. 2014; 3:154–63.

2. Rojeski MT, Gharib H. Nodular thyroid disease. Evaluation and management. N Engl J Med. 1985; 313:428–36.
3. Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ, Cooper DS. The diagnosis and management of thyroid nodules: a review. JAMA. 2018; 319:914–24.
4. Smith MD, Serpell JW, Morgan JL, Cheng MS. Fine needle aspiration in the management of benign thyroid cysts. ANZ J Surg. 2003; 73:477–9.

5. Choi WJ, Baek JH, Choi YJ, Lee JH, Ha EJ, Lee WC, et al. Management of cystic or predominantly cystic thyroid nodules: role of simple aspiration of internal fluid. Endocr Res. 2015; 40:215–9.

6. Ferreira MC, Piaia C, Cadore AC. Percutaneous ethanol injection versus conservative treatment for benign cystic and mixed thyroid nodules. Arch Endocrinol Metab. 2016; 60:211–6.

7. Zingrillo M, Torlontano M, Chiarella R, Ghiggi MR, Nirchio V, Bisceglia M, et al. Percutaneous ethanol injection may be a definitive treatment for symptomatic thyroid cystic nodules not treatable by surgery: five-year follow-up study. Thyroid. 1999; 9:763–7.

8. Sung JY, Kim YS, Choi H, Lee JH, Baek JH. Optimum first-line treatment technique for benign cystic thyroid nodules: ethanol ablation or radiofrequency ablation? AJR Am J Roentgenol. 2011; 196:W210–4.

9. Gong X, Wang F, Du H, Chen X, Shi B. Comparison of ultrasound-guided percutaneous polidocanol injection versus percutaneous ethanol injection for treatment of benign cystic thyroid nodules. J Ultrasound Med. 2018; 37:1423–9.

10. Sung JY, Baek JH, Kim KS, Lee D, Yoo H, Kim JK, et al. Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology. 2013; 269:293–300.

11. Bennedbaek FN, Hegedus L. Treatment of recurrent thyroid cysts with ethanol: a randomized double-blind controlled trial. J Clin Endocrinol Metab. 2003; 88:5773–7.

12. Kim JH, Lee HK, Lee JH, Ahn IM, Choi CG. Efficacy of sonographically guided percutaneous ethanol injection for treatment of thyroid cysts versus solid thyroid nodules. AJR Am J Roentgenol. 2003; 180:1723–6.

13. Baek JH, Ha EJ, Choi YJ, Sung JY, Kim JK, Shong YK. Radiofrequency versus ethanol ablation for treating predominantly cystic thyroid nodules: a randomized clinical trial. Korean J Radiol. 2015; 16:1332–40.

14. Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedus L, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: 2016 update. Endocr Pract. 2016; 22:622–39.
15. Shiina S, Tagawa K, Unuma T, Takanashi R, Yoshiura K, Komatsu Y, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer. 1991; 68:1524–30.

16. Albanese G, Kondo KL. Pharmacology of sclerotherapy. Semin Intervent Radiol. 2010; 27:391–9.

17. Ahmed M, Brace CL, Lee FT Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011; 258:351–69.

18. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.

19. Papini E, Monpeyssen H, Frasoldati A, Hegedus L. 2020 European Thyroid Association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020; 9:172–85.

20. Feldkamp J, Grunwald F, Luster M, Lorenz K, Vorlander C, Fuhrer D. Non-surgical and non-radioiodine techniques for ablation of benign thyroid nodules: consensus statement and recommendation. Exp Clin Endocrinol Diabetes. 2020; 128:687–92.

21. Kim JH, Baek JH, Lim HK, Ahn HS, Baek SM, Choi YJ, et al. 2017 Thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018; 19:632–55.

22. Hahn SY, Shin JH, Na DG, Ha EJ, Ahn HS, Lim HK, et al. Ethanol ablation of the thyroid nodules: 2018 consensus statement by the Korean Society of Thyroid Radiology. Korean J Radiol. 2019; 20:609–20.

23. Jeong WK, Baek JH, Rhim H, Kim YS, Kwak MS, Jeong HJ, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008; 18:1244–50.

24. Lim HK, Lee JH, Ha EJ, Sung JY, Kim JK, Baek JH. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013; 23:1044–9.

25. Aysan E, Idiz UO, Akbulut H, Elmas L. Single-session radiofrequency ablation on benign thyroid nodules: a prospective single center study : radiofrequency ablation on thyroid. Langenbecks Arch Surg. 2016; 401:357–63.

26. Kim YS, Rhim H, Tae K, Park DW, Kim ST. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid. 2006; 16:361–7.

27. Khalilzadeh O, Baerlocher MO, Shyn PB, Connolly BL, Devane AM, Morris CS, et al. Proposal of a new adverse event classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol. 2017; 28:1432–7.

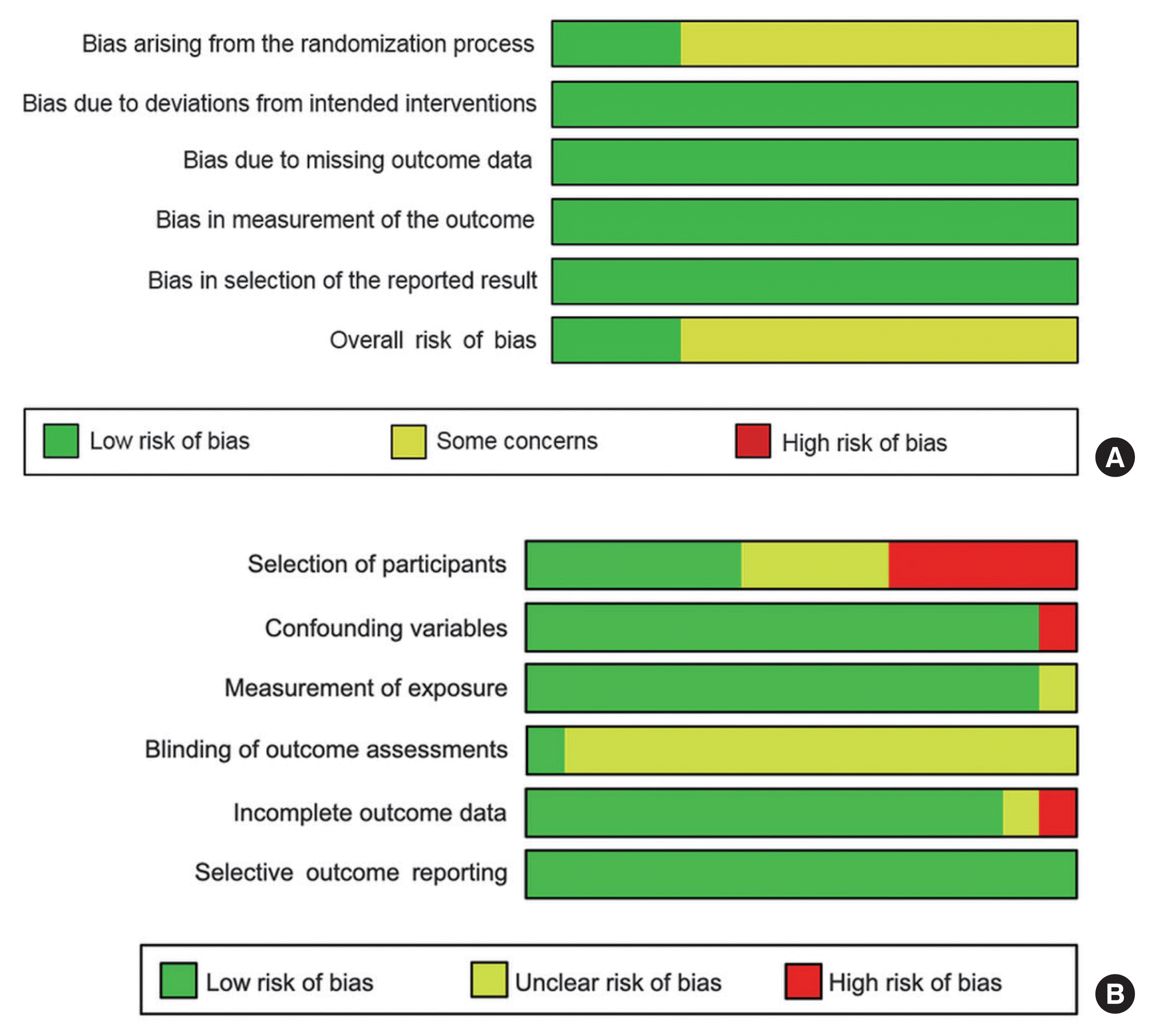
28. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898.

29. Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013; 66:408–14.

30. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–60.

31. Valcavi R, Frasoldati A. Ultrasound-guided percutaneous ethanol injection therapy in thyroid cystic nodules. Endocr Pract. 2004; 10:269–75.

32. Verde G, Papini E, Pacella CM, Gallotti C, Delpiano S, Strada S, et al. Ultrasound guided percutaneous ethanol injection in the treatment of cystic thyroid nodules. Clin Endocrinol (Oxf). 1994; 41:719–24.

33. Cho YS, Lee HK, Ahn IM, Lim SM, Kim DH, Choi CG, et al. Sonographically guided ethanol sclerotherapy for benign thyroid cysts: results in 22 patients. AJR Am J Roentgenol. 2000; 174:213–6.
34. Guglielmi R, Pacella CM, Bianchini A, Bizzarri G, Rinaldi R, Graziano FM, et al. Percutaneous ethanol injection treatment in benign thyroid lesions: role and efficacy. Thyroid. 2004; 14:125–31.

35. Jang SW, Baek JH, Kim JK, Sung JY, Choi H, Lim HK, et al. How to manage the patients with unsatisfactory results after ethanol ablation for thyroid nodules: role of radiofrequency ablation. Eur J Radiol. 2012; 81:905–10.

36. Perez CL, Fighera TM, Miasaki F, Mesa Junior CO, Paz Filho GJ, Graf H, et al. Evaluation of percutaneous ethanol injections in benign thyroid nodules. Arq Bras Endocrinol Metabol. 2014; 58:912–7.

37. Negro R, Colosimo E, Greco G. Outcome, pain perception, and health-related quality of life in patients submitted to percutaneous ethanol injection for simple thyroid cysts. J Thyroid Res. 2017; 2017:9536479.

38. Espenbetova M, Amrenova K, Zhumanbayeva Z, Zamanbekova Z, Yurkovskaya O, Shalgumbayeva G, et al. Effectiveness of percutaneous ethanol injection in nodular diseases of the thyroid gland: 10-year follow-up. Endoc Pract. 2018; 24:982–7.

39. Ozderya A, Aydin K, Gokkaya N, Temizkan S. Percutaneous ethanol injection for benign cystic and mixed thyroid nodules. Endocr Pract. 2018; 24:548–55.

40. Halenka M, Karasek D, Schovanek J, Frysak Z. Safe and effective percutaneous ethanol injection therapy of 200 thyroid cysts. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020; 164:161–7.

41. Reverter JL, Alonso N, Avila M, Lucas A, Mauricio D, Puig-Domingo M. Evaluation of efficacy, safety, pain perception and health-related quality of life of percutaneous ethanol injection as first-line treatment in symptomatic thyroid cysts. BMC Endocr Disord. 2015; 15:73.

42. Park HS, Yim Y, Baek JH, Choi YJ, Shong YK, Lee JH. Ethanol ablation as a treatment strategy for benign cystic thyroid nodules: a comparison of the ethanol retention and aspiration techniques. Ultrasonography. 2019; 38:166–71.

43. Friedrich JO, Adhikari NK, Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol. 2007; 7:5.

44. Lee GM, You JY, Kim HY, Chai YJ, Kim HK, Dionigi G, et al. Successful radiofrequency ablation strategies for benign thyroid nodules. Endocrine. 2019; 64:316–21.

45. Kim YJ, Baek JH, Ha EJ, Lim HK, Lee JH, Sung JY, et al. Cystic versus predominantly cystic thyroid nodules: efficacy of ethanol ablation and analysis of related factors. Eur Radiol. 2012; 22:1573–8.

46. Hegedus L, Hansen JM, Karstrup S, Torp-Pedersen S, Juul N. Tetracycline for sclerosis of thyroid cysts. A randomized study. Arch Intern Med. 1988; 148:1116–8.

47. Kalra N, Ahuja CK, Dutta P, Rajwanshi A, Mittal BR, Bhansali A, et al. Comparison of sonographically guided percutaneous sodium tetradecyl sulfate injection with ethanol injection in the treatment of benign nonfunctioning thyroid nodules. J Vasc Interv Radiol. 2014; 25:1218–24.

48. Dong Y, Zhou J, Liu Z, Luo T, Zhan W. Efficacy assessment of ultrasound guided lauromacrogol injection for ablation of benign cystic and predominantly cystic thyroid nodules. Front Pharmacol. 2019; 10:478.

49. Yuce G, Ates OF, Polat B, Genc B, Canyigit M. Ablation of cystic thyroid nodules with n-butyl cyanoacrylate: a preliminary study. Endocr Pract. 2020; 26:492–8.

50. In HS, Kim DW, Choo HJ, Jung SJ, Kang T, Ryu JH. Ethanol ablation of benign thyroid cysts and predominantly cystic thyroid nodules: factors that predict outcome. Endocrine. 2014; 46:107–13.

51. Suh CH, Baek JH, Ha EJ, Choi YJ, Lee JH, Kim JK, et al. Ethanol ablation of predominantly cystic thyroid nodules: evaluation of recurrence rate and factors related to recurrence. Clin Radiol. 2015; 70:42–7.

52. Kim DW, Rho MH, Kim HJ, Kwon JS, Sung YS, Lee SW. Percutaneous ethanol injection for benign cystic thyroid nodules: is aspiration of ethanol-mixed fluid advantageous? AJNR Am J Neuroradiol. 2005; 26:2122–7.
53. Lee SJ, Ahn IM. Effectiveness of percutaneous ethanol injection therapy in benign nodular and cystic thyroid diseases: long-term follow-up experience. Endocr J. 2005; 52:455–62.

54. Solymosi T. Percutaneous ethanol injection efficacy in the treatment of benign thyroid nodules. Ten-year follow-up of 254 patients. Orv Hetil. 2020; 161:224–31.
55. Monzani F, Lippi F, Goletti O, Del Guerra P, Caraccio N, Lippolis PV, et al. Percutaneous aspiration and ethanol sclerotherapy for thyroid cysts. J Clin Endocrinol Metab. 1994; 78:800–2.

56. Lee JH, Kim YS, Lee D, Choi H, Yoo H, Baek JH. Radiofrequency ablation (RFA) of benign thyroid nodules in patients with incompletely resolved clinical problems after ethanol ablation (EA). World J Surg. 2010; 34:1488–93.

57. Kim DW. Sonography-guided ethanol ablation of a remnant solid component after radio-frequency ablation of benign solid thyroid nodules: a preliminary study. AJNR Am J Neuroradiol. 2012; 33:1139–43.

58. Park HS, Baek JH, Choi YJ, Lee JH. Innovative techniques for image-guided ablation of benign thyroid nodules: combined ethanol and radiofrequency ablation. Korean J Radiol. 2017; 18:461–9.

59. Baek JH, Lee JH, Sung JY, Bae JI, Kim KT, Sim J, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012; 262:335–42.

60. Mauz PS, Maassen MM, Braun B, Brosch S. How safe is percutaneous ethanol injection for treatment of thyroid nodule? Report of a case of severe toxic necrosis of the larynx and adjacent skin. Acta Otolaryngol. 2004; 124:1226–30.

61. Park NH, Kim DW, Park HJ, Lee EJ, Park JS, Park SI, et al. Thyroid cysts treated with ethanol ablation can mimic malignancy during sonographic follow-up. J Clin Ultrasound. 2011; 39:441–6.

62. Park JS, Kim DW, Eun CK, Choi SJ, Rho MH. Long-term follow-up sonography of benign cystic thyroid nodules after a percutaneous ethanol injection: the incidence of malignancy-mimicking nodules. J Korean Radiol Soc. 2008; 58:21–8.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download