INTRODUCTION
MATERIALS AND METHODS
Materials
Distilled water (DW) extraction
70% ethanol extraction
95% hexane extraction
Cell culture and treatments
Immunofluorescence (IF)
Animal treatments and experimental design
Hematoxylin and eosin (H&E) staining
Immunohistochemistry
Western blotting assay
Serum Concentrations of DHT
Statistical analysis
RESULTS
Comparison of AR signal inhibitory effect by extraction methods of A. distichum
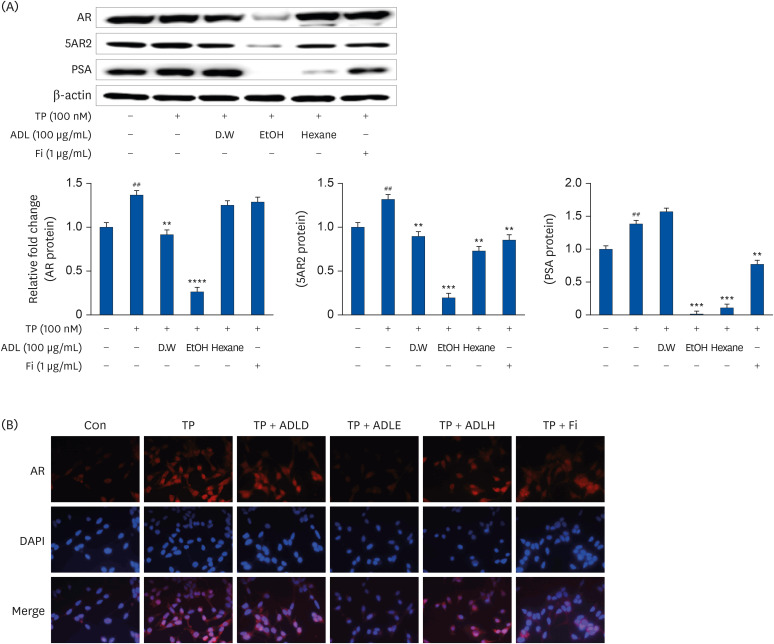 | Fig. 1Comparison of the AR signal inhibitory effect by extraction methods of A. distichum. (A) Representative western blot showing the bands of AR, 5AR2, and PSA. LNCaP cells were incubated for 24 h in a culture medium containing TP (100 nmol), ADL (A. distichum leaves, 100 μg/mL; DW, 70% EtOH or 95% hexane), or Fi (1 μg/mL). (B) IF detection of AR in LNCaP cells. LNCaP cells were plated on glass slides and processed for IF. The cells were stained with the anti-AR antibody and then with AlexaFluor 588 secondary antibody. Nuclear staining was done by DAPI staining. Image merging of AR and DAPI was analyzed with a Zeiss fluorescence microscope program.AR, androgen receptor; TP, testosterone propionate; 5AR2, 5α-reductase type2; PSA, prostate specific antigen; ADL, A. distichum leaf; DW, distilled water; EtOH, ethanol; Fi, finasteride; IF, immunofluorescence; ADLD, A. distichum leaf distilled water extract; ADLE, A. distichum leaf ethanol extract; ADLH, A. distichum leaf hexane extract; DAPI, 4′,6-diamidino-2-phenylindole.
##P < 0.01 compared to untreated LNCaP cell; **P < 0.01, ***P < 0.001 and ****P < 0.0001 vs. TP-treated LNCaP cell.
|
Effect of ADLE on the inhibition of AR signaling according to harvest season
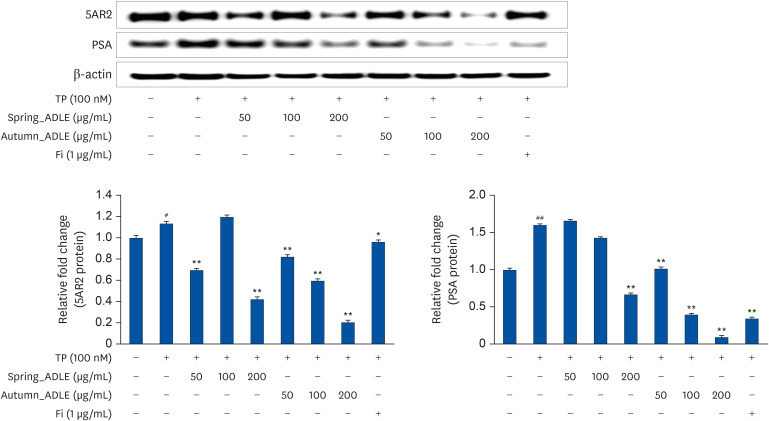 | Fig. 2Effect of ADLE on inhibiting androgen receptor signaling according to harvest season. LNCaP cells were incubated for 24 h in culture medium containing TP (100 nmol), spring_ADLE (50, 100, or 200 μg/mL), autumn_ADLE (50, 100, or 200 μg/mL) or Fi (1 μg/mL). Thereafter, cell lysates (30 μg) were analyzed for the expression of 5AR2 and PSA. The relative protein expression levels were normalized to those of β-actin.ADLE, A. distichum leaf ethanol extract; TP, testosterone propionate; Fi, finasteride; 5AR2, 5α-reductase type2; PSA, prostate specific antigen.
#P < 0.05 and ##P < 0.01 vs. untreated cells; *P < 0.05 and **P < 0.01 vs. TP-treated cells.
|
Effect of ADLE on prostate tissue weight in BPH rats
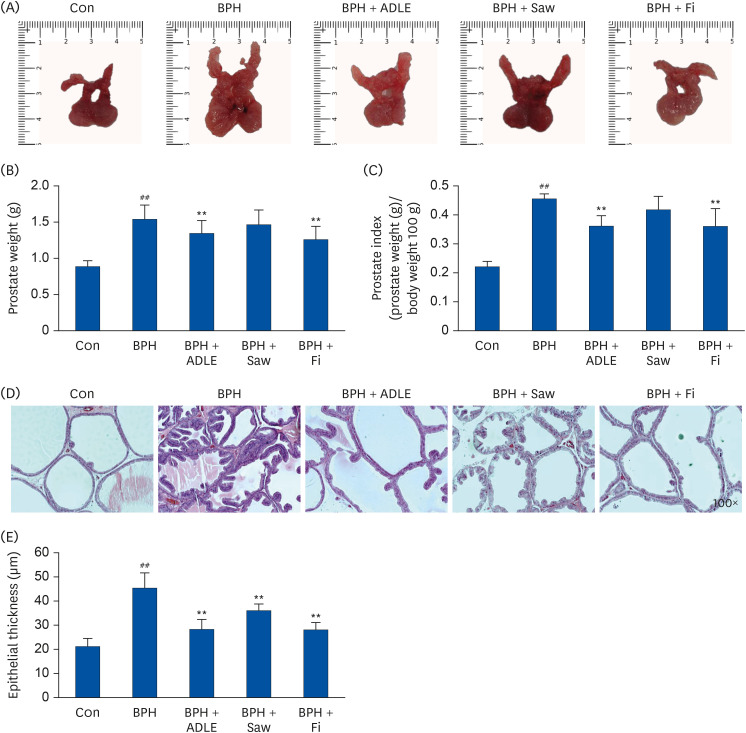 | Fig. 3Effect of ADLE on prostate tissue in TP-induced BPH rats. (A) Photographs of the prostate tissues (VP, DLP, AP). (B) Total prostate tissue weight and (C) prostate indices of the rats. (D) H&E-stained prostate tissues (magnification 100×). (E) Epithelial thickness of the prostate tissues.ADLE, A. distichum leaf ethanol extract; TP, testosterone propionate; BPH, benign prostatic hyperplasia; VP, ventral prostate; DLP, dorsolateral prostate; AP, anterior prostate; Fi, finasteride; H&E, hematoxylin and eosin; DW, distilled water; Con, corn oil subcutaneous injection and DW oral intake; BPH, TP subcutaneous injection (3 mg/kg) and oral intake; BPH + ADLE, TP subcutaneous injection (3 mg/kg) and A. distichum leaves 70% ethanol extract (100 mg/kg) oral intake; BPH + Saw, TP subcutaneous injection (3 mg/kg) and saw palmetto extract (100 mg/kg) oral intake; BPH + Fi, subcutaneous injection and finasteride (1 mg/kg) oral intake.
##P < 0.01 vs. the Con group; **P < 0.01 vs. the BPH group (n = 8 per group).
|
Effect of ADLE on AR-signaling-related factors in prostate tissues of BPH rats
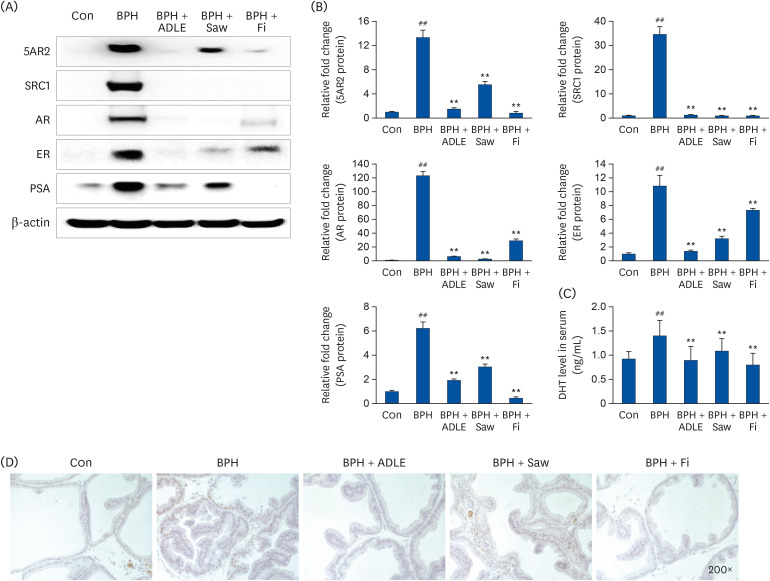 | Fig. 4Effect of ADLE on the AR-signaling-related factors in prostate tissues of BPH rats. (A) Representative western blot showing the bands of 5AR2, SRC1, AR, ER, and PSA. (B) densitometer of the protein expression level. (C) Concentration of DHT in the serum. (D) Immunochemical staining of AR in prostate tissue.ADLE, A. distichum leaf ethanol extract; TP, testosterone propionate; AR, androgen receptor; BPH, benign prostatic hyperplasia; 5AR2, 5α-reductase type2; SRC1, steroid receptor co-activator 1; ER, estrogen receptor; PSA, prostate specific antigen; DHT, dihydrotestosterone; DW, distilled water; Con, corn oil subcutaneous injection and DW oral intake; BPH, TP subcutaneous injection (3 mg/kg) and oral intake; BPH + ADLE, TP subcutaneous injection (3 mg/kg) and A. distichum leaves 70% ethanol extract (100 mg/kg) oral intake; BPH + Saw, TP subcutaneous injection (3 mg/kg) and saw palmetto extract (100 mg/kg) oral intake; BPH + Fi, subcutaneous injection and finasteride (1 mg/kg) oral intake.
##P < 0.01 vs. the Con group; **P < 0.01 vs. the BPH group (n = 8 per group).
|
Effect of ADLE on the growth factors and PI3K/AKT pathway in prostate tissues of BPH rats
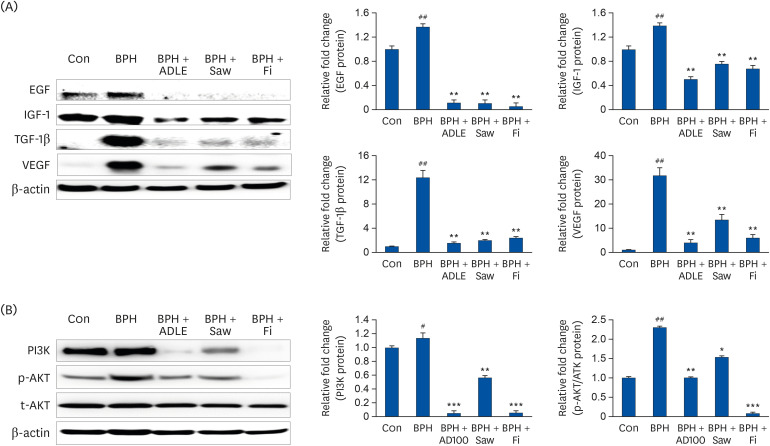 | Fig. 5Expression of growth factors and PI3K/AKT in prostate tissue after the administration of ADLE in BPH. (A) Representative western blot showing the bands of EGF, IGF-1, TGF-β1, and VEGF. (B) Representative western blot showing the bands of PI3K, p-AKT and t-AKT.TP, testosterone propionate; ADLE, A. distichum leaf ethanol extract; BPH, benign prostatic hyperplasia; EGF, epidermal growth factor; IGF-1, insulin-like growth factor-1; VEGF, vascular endothelial growth factor; TGF-β1, transforming growth factor-beta 1; DW, distilled water; Con, corn oil subcutaneous injection and DW oral intake; BPH, TP subcutaneous injection (3 mg/kg) and oral intake; BPH + ADLE, TP subcutaneous injection (3 mg/kg) and A. distichum leaves 70% ethanol extract (100 mg/kg) oral intake; BPH + Saw, TP subcutaneous injection (3 mg/kg) and saw palmetto extract (100 mg/kg) oral intake; BPH + Fi, subcutaneous injection and finasteride (1 mg/kg) oral intake.
#P < 0.05 and ##P < 0.01 vs. the Con group; *P < 0.05, **P < 0.01 and ***P < 0.001 vs. the BPH group (n = 8 per group).
|
Effect of ADLE on the proliferation and apoptosis-related factor in prostate tissues of BPH rats
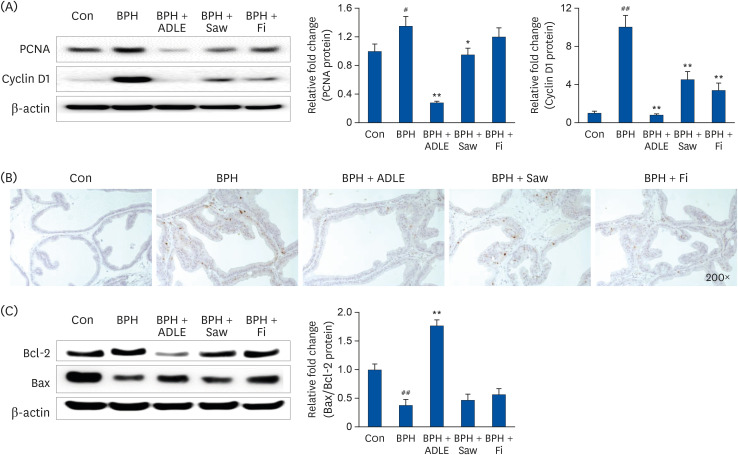 | Fig. 6Expression of proliferation and apoptosis-related factor in the prostate tissue after the administration of ADLE in BPH. (A) Representative western blot showing the bands of PCNA and cyclin D1. (B) Immunochemical staining of AR in prostate tissue. (C) Representative western blot showing the bands of Bcl-2 and Bax.ADLE, A. distichum leaf ethanol extract; BPH, benign prostatic hyperplasia; PCNA, proliferative cell nuclear antigen; AR, androgen receptor; DW, distilled water; Con, corn oil subcutaneous injection and DW oral intake; BPH, TP subcutaneous injection (3 mg/kg) and oral intake; BPH + ADLE, TP subcutaneous injection (3 mg/kg) and A. distichum leaves 70% ethanol extract (100 mg/kg) oral intake; BPH + Saw, TP subcutaneous injection (3 mg/kg) and saw palmetto extract (100 mg/kg) oral intake; BPH + Fi, subcutaneous injection and finasteride (1 mg/kg) oral intake.
#P < 0.05 and ##P < 0.01 vs. the Con group; *P< 0.05 and **P < 0.01 vs. the BPH group (n = 8 per group).
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download