1. Mills JA, Marks E, Reynolds T, Cieza A. Rehabilitation: essential along the continuum of care. Jamison DT, Gel-band H, Horton S, Jha P, Laxminarayan R, Mock CN, editors. Disease control priorities: improving health and reducing poverty. 3rd ed. Washington, DC: The International Bank for Reconstruction and Development;2017. p. 285–95.

2. Colbenson GA, Johnson A, Wilson ME. Post-intensive care syndrome: impact, prevention, and management. Breathe (Sheff). 2019; 15:98–101.

3. Lui SK, Nguyen MH. Elderly stroke rehabilitation: overcoming the complications and its associated challenges. Curr Gerontol Geriatr Res. 2018; 2018:9853837.

4. Bellmann B, Lin T, Greissinger K, Rottner L, Rillig A, Zimmerling S. The beneficial effects of cardiac rehabilitation. Cardiol Ther. 2020; 9:35–44.

5. Al Moamary MS, Alorainy H, Al-Hajjaj MS. Pulmonary rehabilitation: a regional perspective evidenced-based review. Ann Thorac Med. 2014; 9:3–7.

6. Troosters T, Gayan-Ramirez G, Pitta F, Gosselin N, Gosselink R, Decramer M. Exercise effort training for COPD: physiological basis and results. Rev Mal Respir. 2004; 21:319–27.
7. Ryrso CK, Godtfredsen NS, Kofod LM, Lavesen M, Mogensen L, Tobberup R, et al. Lower mortality after early supervised pulmonary rehabilitation following COPD-exacerbations: a systematic review and meta-analysis. BMC Pulm Med. 2018; 18:154.

8. Dowman L, Hill CJ, Holland AE. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev. 2014; (10):CD006322.

9. Tiep B, Sun V, Koczywas M, Kim J, Raz D, Hurria A, et al. Pulmonary rehabilitation and palliative care for the lung cancer patient. J Hosp Palliat Nurs. 2015; 17:462–8.

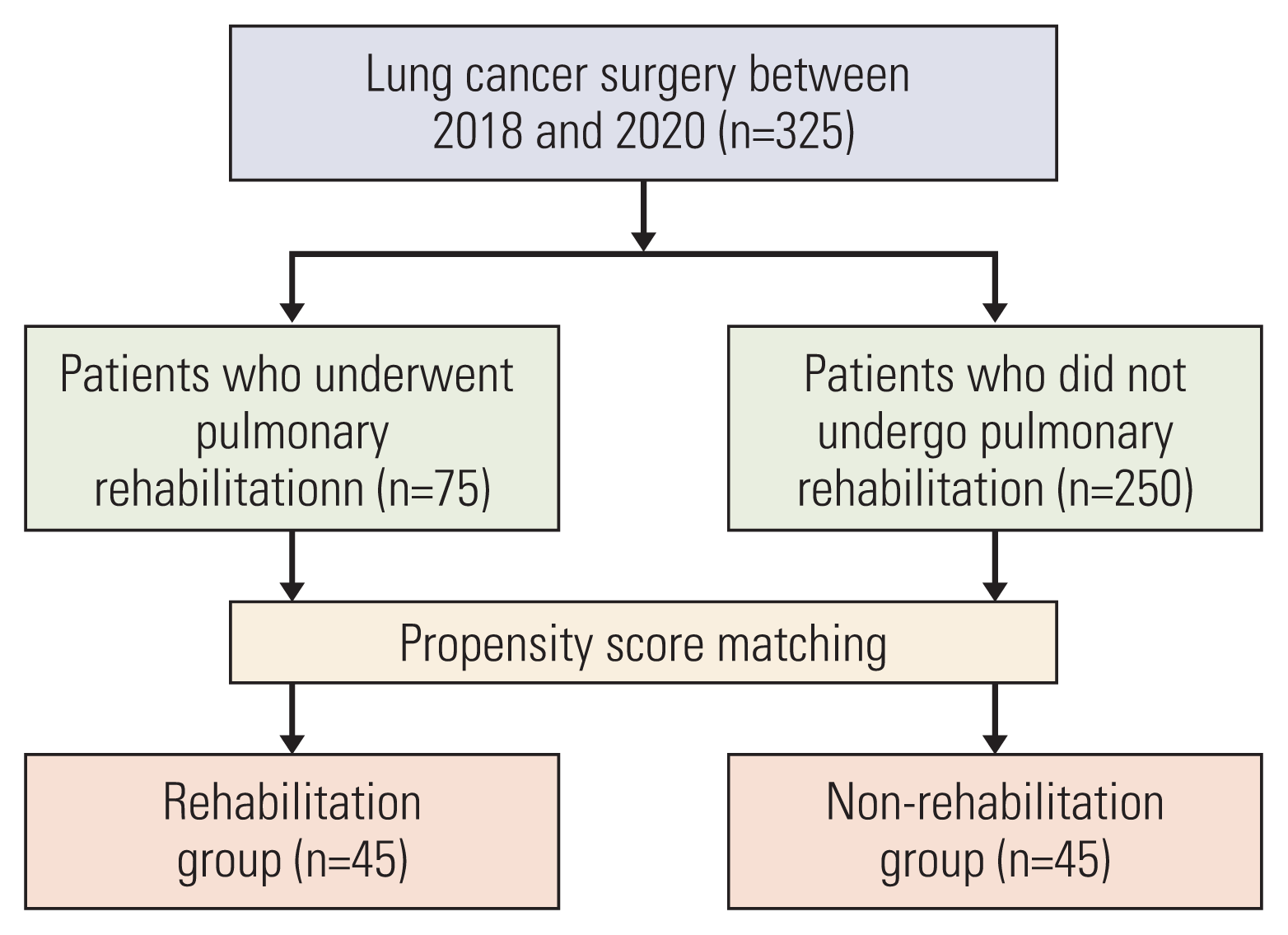
10. Bradley A, Marshall A, Stonehewer L, Reaper L, Parker K, Bevan-Smith E, et al. Pulmonary rehabilitation programme for patients undergoing curative lung cancer surgery. Eur J Cardiothorac Surg. 2013; 44:e266–71.

11. Vagvolgyi A, Rozgonyi Z, Kerti M, Agathou G, Vadasz P, Varga J. Effectiveness of pulmonary rehabilitation and correlations in between functional parameters, extent of thoracic surgery and severity of post-operative complications: randomized clinical trial. J Thorac Dis. 2018; 10:3519–31.

12. Pehlivan E, Balci A, Kilic L. Can functional inoperability in lung cancer patients be changed by pulmonary rehabilitation? Turk Gogus Kalp Damar Cerrahisi Derg. 2019; 27:212–8.
13. Arnold MT, Dolezal BA, Cooper CB. Pulmonary rehabilitation for chronic obstructive pulmonary disease: highly effective but often overlooked. Tuberc Respir Dis (Seoul). 2020; 83:257–67.

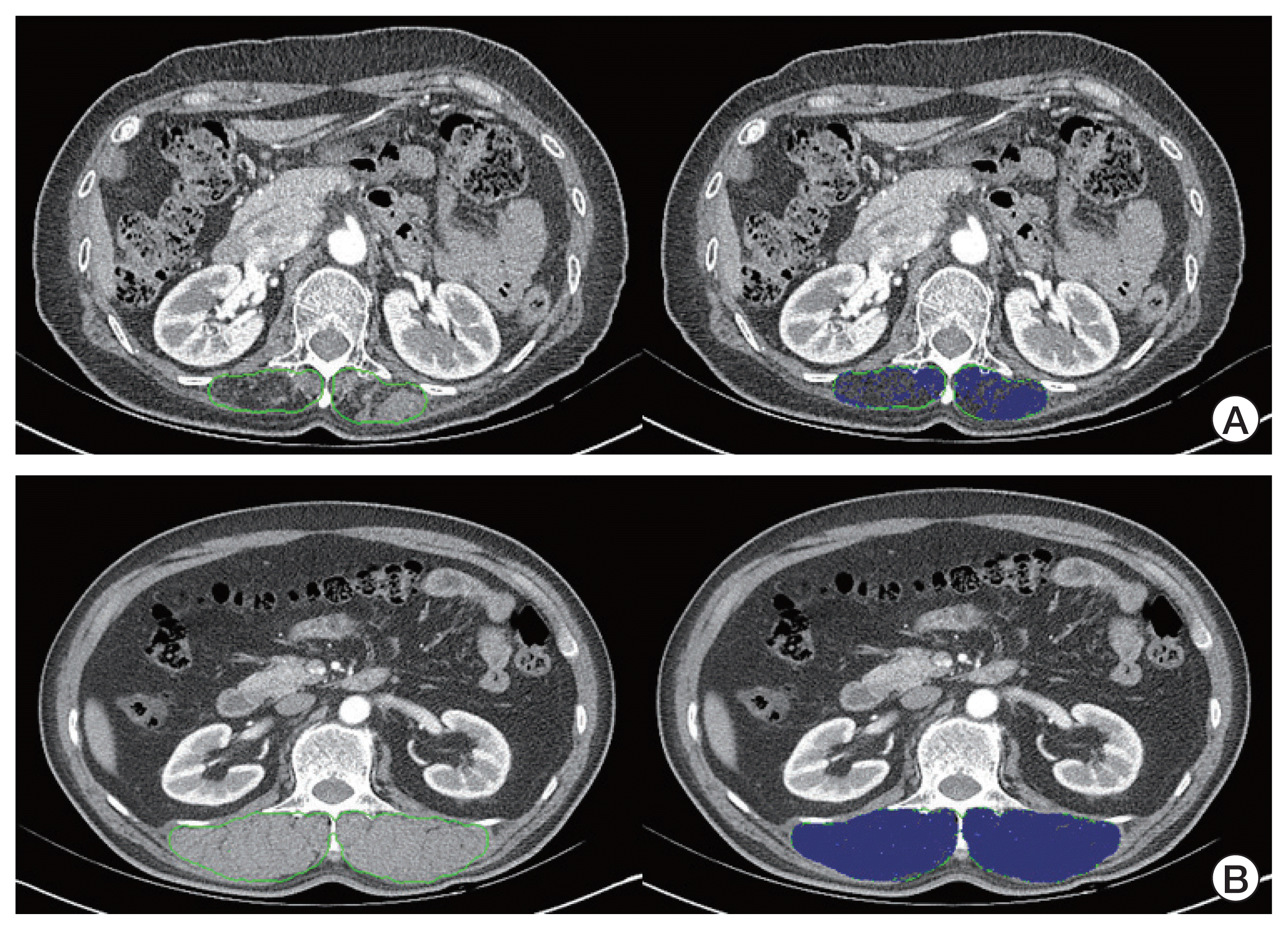
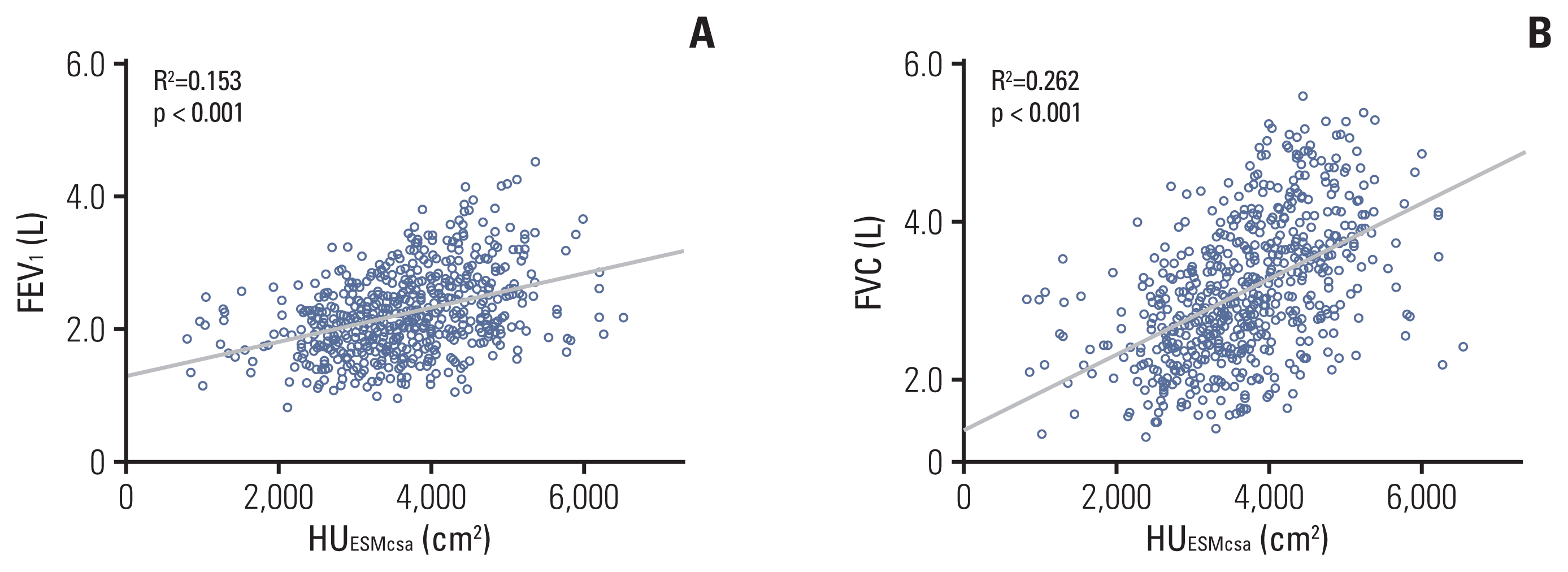
14. Bak SH, Kwon SO, Han SS, Kim WJ. Computed tomography-derived area and density of pectoralis muscle associated disease severity and longitudinal changes in chronic obstructive pulmonary disease: a case control study. Respir Res. 2019; 20:226.

15. Tanimura K, Sato S, Fuseya Y, Hasegawa K, Uemasu K, Sato A, et al. Quantitative assessment of erector spinae muscles in patients with chronic obstructive pulmonary disease: novel chest computed tomography-derived index for prognosis. Ann Am Thorac Soc. 2016; 13:334–41.

16. Sanders KJC, Degens J, Dingemans AC, Schols A. Cross-sectional and longitudinal assessment of muscle from regular chest computed tomography scans: L1 and pectoralis muscle compared to L3 as reference in non-small cell lung cancer. Int J Chron Obstruct Pulmon Dis. 2019; 14:781–9.
17. Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf). 2014; 210:489–97.

18. Ewaschuk JB, Almasud A, Mazurak VC. Role of n-3 fatty acids in muscle loss and myosteatosis. Appl Physiol Nutr Metab. 2014; 39:654–62.

19. Hill K, Vogiatzis I, Burtin C. The importance of components of pulmonary rehabilitation, other than exercise training, in COPD. Eur Respir Rev. 2013; 22:405–13.

20. Wouters EF, Posthuma R, Koopman M, Liu WY, Sillen MJ, Hajian B, et al. An update on pulmonary rehabilitation techniques for patients with chronic obstructive pulmonary disease. Expert Rev Respir Med. 2020; 14:149–61.

21. Wilson JS, O’Neill B, Reilly J, MacMahon J, Bradley JM. Education in pulmonary rehabilitation: the patient’s perspective. Arch Phys Med Rehabil. 2007; 88:1704–9.

22. Aldhahir AM, Rajeh AM, Aldabayan YS, Drammeh S, Subbu V, Alqahtani JS, et al. Nutritional supplementation during pulmonary rehabilitation in COPD: a systematic review. Chron Respir Dis. 2020; 17:1479973120904953.

23. Popa-Velea O, Purcarea VL. Psychological intervention: a critical element of rehabilitation in chronic pulmonary diseases. J Med Life. 2014; 7:274–81.
24. Rispoli M, Salvi R, Cennamo A, Di Natale D, Natale G, Meoli I, et al. Effectiveness of home-based preoperative pulmonary rehabilitation in COPD patients undergoing lung cancer resection. Tumori. 2020; 106:203–11.

25. Moon SW, Choi JS, Lee SH, Jung KS, Jung JY, Kang YA, et al. Thoracic skeletal muscle quantification: low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir Res. 2019; 20:35.

26. Benzo R, Wigle D, Novotny P, Wetzstein M, Nichols F, Shen RK, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. 2011; 74:441–5.

27. Choi MG, Lee HY, Song SY, Kim SS, Lee SH, Kim W, et al. The effects of simultaneous pulmonary rehabilitation during thoracic radiotherapy in the treatment of malignant diseases. Tuberc Respir Dis. 2021; 84:148–58.

28. Ahn HJ, Jeon JY, Kim SS, Song SY, Kim W, Lee SW, et al. Feasibility of an outpatient-based pulmonary rehabilitation program for lung cancer patients during radiation therapy. Thorac Cancer. 2021; 12:2241–6.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download