INTRODUCTION
Coronavirus disease 2019 (COVID-19) has rapidly spread around the globe since its discovery as a case of pneumonia of unknown cause in China in December 2019.
1 Since the World Health Organization (WHO) declared COVID-19 a pandemic in March 2020,
2 several variants of concern have emerged, leading to nearly 470 million confirmed cases and more than 6 million deaths as of March 22, 2022.
3
While most patients suffer from a mild febrile disease, many patients progress to require hospitalization and respiratory assistance, such as high-flow nasal cannula (HFNC) oxygen therapy or mechanical ventilation (MV), and even to death.
4 Attempts to predict mortality have been made since the early days of the pandemic, and studies have shown that old age is the most evident risk factor of severe infection and mortality and that underlying diseases are also a risk factor.
4567 While severe infections and death occur even in relatively young patients, reports on the clinical features and prognosis of COVID-19 infection in these patients are limited.
8910 Among the younger generations, obesity (body mass index [BMI] > 30 kg/m
2) as well as pre-existing conditions have been identified as key risk factors, and mechanisms such as cytokine storm have been described.
11
Since the advent of variant viruses, such as the delta variant,
12 there has been a fourth wave of COVID-19, and the rate of severe COVID-19 cases among young patients aged 50 years or younger has increased in Korea. However, detailed data on the characteristics, clinical manifestations, and treatment outcomes of these patients and their differences with older patients are lacking in Korea. Identifying the clinical characteristics and mortality risk factors in young critical patients would help to lower the mortality rate in this patient population.
This study aims to analyze the clinical characteristics and mortality risk factors of young patients (≤ 50 years) with critical COVID-19 infection (defined as receiving respiratory support such as HFNC or greater levels of oxygen support) and evaluate their differences with older patients above age 50 years in Korea.
DISCUSSION
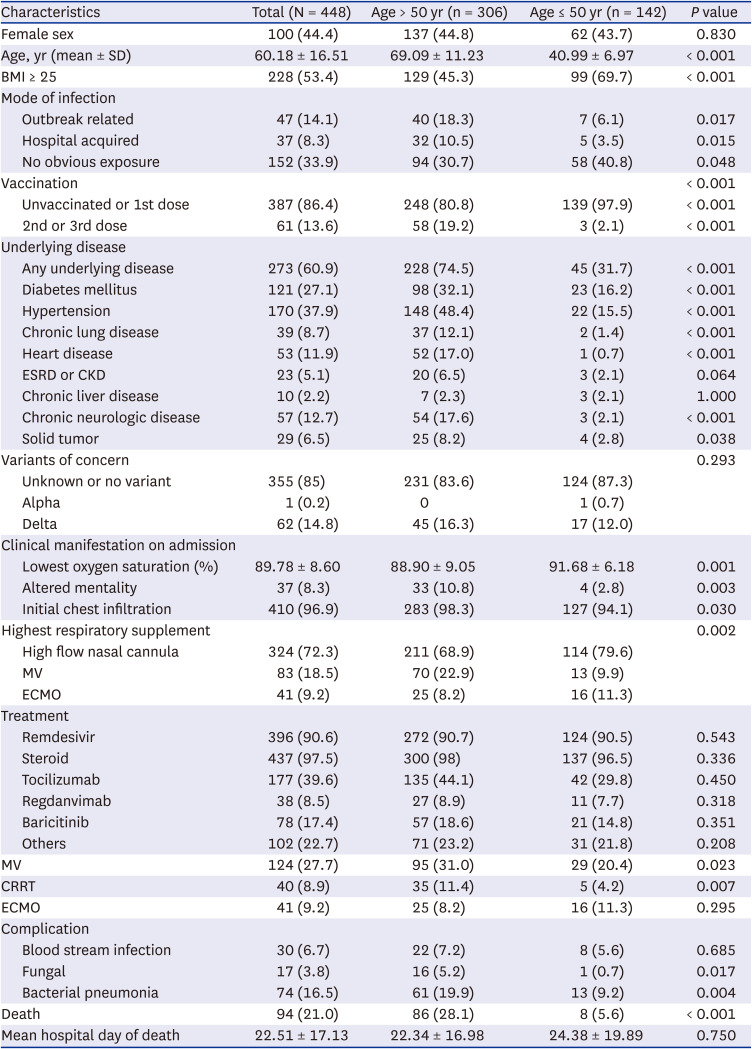
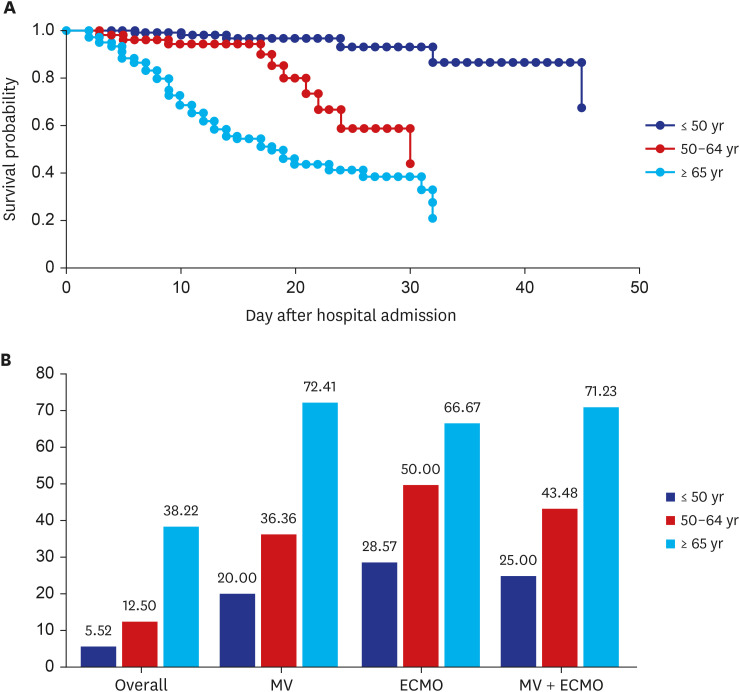
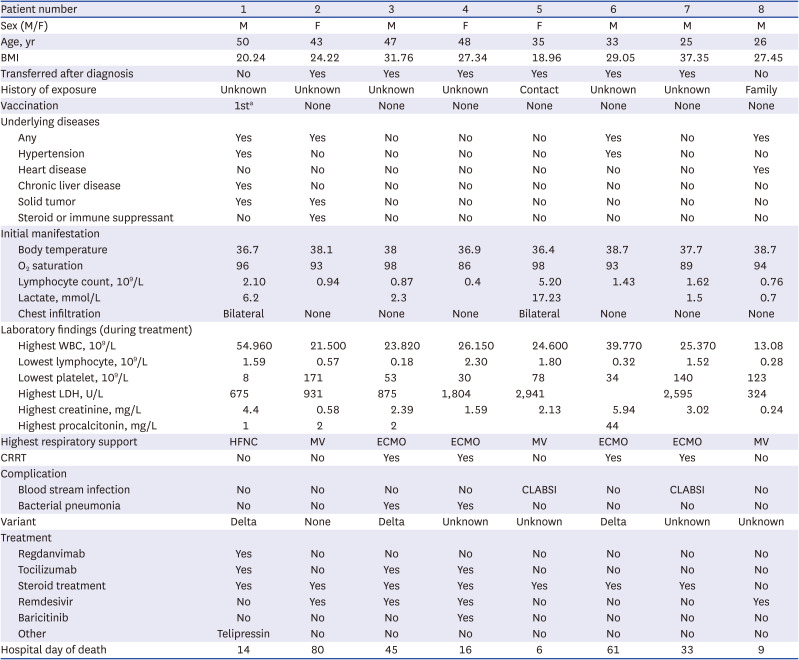
The present study differs from previous studies in that the participants were limited to critical patients on respiratory support (HFNC or greater support) and it analyzed the CFR-S and risk factors for mortality in patients ≤ 50 years of age. The overall CFR-S was 21.0% in our study, markedly higher than the 1.54% reported based on the WHO data.
3 Even by age, the CFR-S was 5.6% in the ≤ 50 years group, 29.3% in the > 50 years group, and 38.22% in the ≥ 65 years group, which are also higher than the values reported (20%) in another study on patients aged 65 years and older.
14 This may be attributable to the fact that this study was only conducted on critical patients placed on HFNC or higher respiratory support, and our findings confirm that CFR-S is markedly high when COVID-19 progresses to a critical case. The CFR-S was even higher in cases that required MV or higher respiratory support, 25% for patients aged 50 years and younger and 71% for 65 years or older. A Chinese study
15 evaluated mortality among young adults showed 25% of CFR-S, which is much higher than those of our study (5.6%). This might be because they included patients younger than 60 years, while 50 years or younger in this study. The mortality would be different between patients aged 50–60 years and younger than 50 years, because age is the most important risk factor for mortality in COVID-19.
14614 Furthermore, the Chinese study was conducted during early period of COVID-19 pandemic, when remdesivir or steroid treatment was not recommended as a standard therapy, while more than 90% of the patients were treated with remdesivir and steroid in our study. This result confirms that remdesivir and steroid treatment has contributed to reduction of case fatality. Nonetheless, CFR-S was irrelevant to age in the young population of this study, while mortality increased with advancing age in the older population.
Critical patients aged 50 years and younger included in our study had a higher BMI than their older (> 50 years) counterparts and had a lower prevalence of pre-existing conditions, and this difference seems to be attributable to the age difference. The proportion of patients having BMI higher than 25 in critical patients under 50 years old was 69.7%, which is two-fold higher than in general population, and this supports that obesity is a risk factor for severe COVID-19 in young adults.
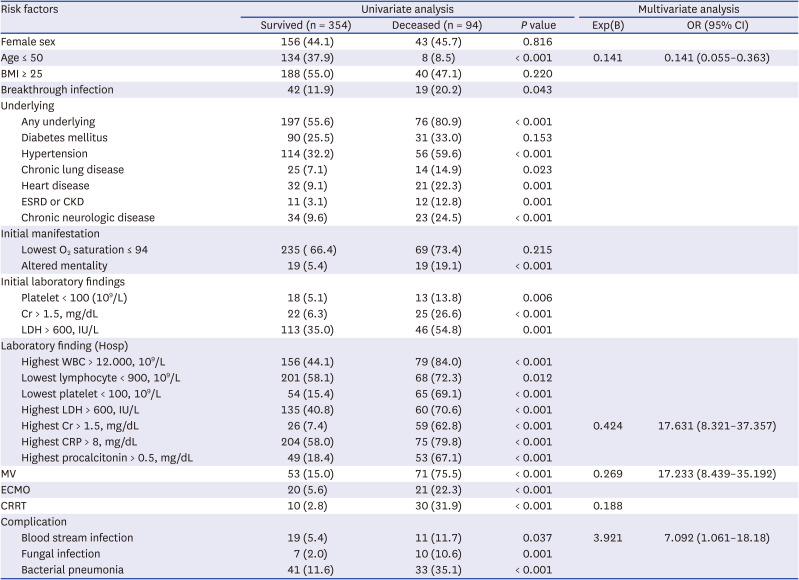
Regarding risk factors associated with mortality, age (95% CI, 11.993–18.919), initial creatinine > 1.5 mg/dL (95% CI, 2.898–10.194), highest creatinine > 1.5 mg/dL (95% CI, 11.855–37.685), lowest platelet count < 100 (10
9/L) (95% CI, 7.268–20.770), use of MV (95% CI, 10.079–30.493), and CRRT (95% CI, 7.513–34.611) were identified as significant independent predictors of mortality in this study. This is similar to the findings of prior studies. A Korean study on adults aged 65 years and over
14 identified HAI, DM, chronic lung disease, chronic neurologic disease, hypoxia, altered mental status, and CRP > 8.0 mg/dL as mortality risk factors, and in our study, these factors were significant mortality risk factors in the univariate analysis but not in the multivariate analysis, presumably because they are common risk factors for both severity and mortality and our study included only critical cases. Some studies reported sex-specific differences in mortality rates,
1617 and some hypothesized this to be influenced by androgen.
16 However, sex was not a significant predictor of mortality in the entire patient population or in the ≤ 50 years and > 50 years groups.
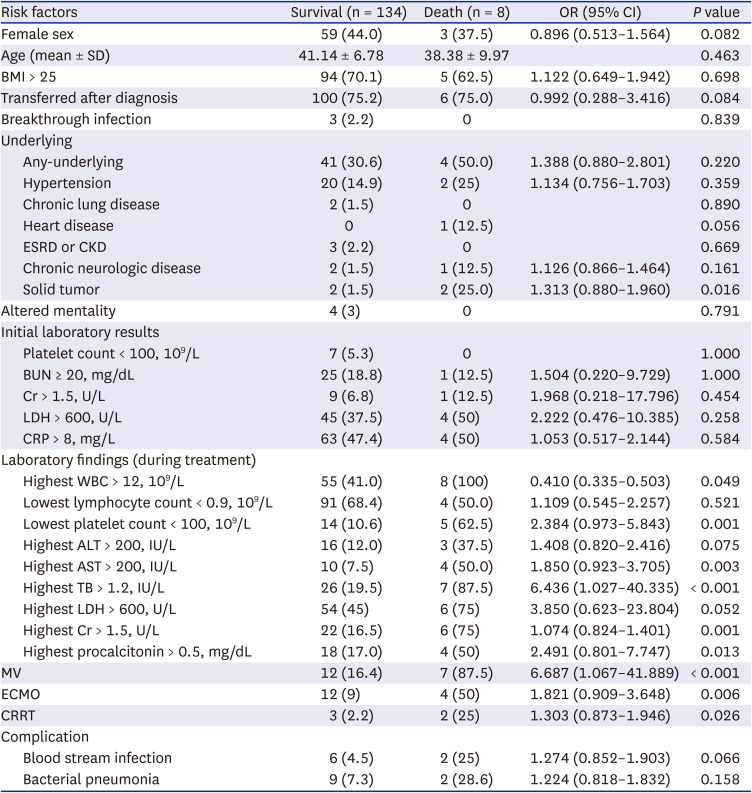
The predictors of mortality in the ≤ 50 years group were similar to those in the total patient population, but solid tumor was another significant risk factor in this group. CFR-S is high among patients with active cancer, so prevention is important for this specific patient population. The CFR-S was high among patients with a pre-existing condition, but the result was not significant due to the small number of deaths. Regarding obesity, 5 out of 8 patients who died in the ≤ 50 years group having a BMI ≥ 25 and two having a BMI ≥ 30. A study that examined 210 patients aged 18–45 years confirmed with COVID-19 in the US
9 reported that BMI ≥ 30 (OR, 6.29; 95% CI, 1.76–22.46,
P = 0.004) as well as MV (OR, 6.01; 95% CI, 2.5–14.48,
P < 0.001) was a statistically significant predictor of mortality, and other studies on children
8 and adults
17 also support the hypothesis that obesity exacerbates COVID-19 infection and increases the risk for mortality. The prevalence of obesity is lower in the Korean population compared to other racial populations; thus, further studies are needed.
One notable finding is that 31 out of 142 patients aged 50 years and younger with critical COVID-19 infection (22%, 19 women) did not have a known risk factor, such as BMI > 25 or a pre-existing condition. This is more distinguishable when compares with the fact that only 14 patients who died in the > 50 years group did not have a pre-existing condition. This shows that even the young, healthy population without a pre-existing condition could—although less likely—progress to a severe case, necessitating effort to prevent the infection in advance.
In this study, patients who had been transferred into the hospital seemed to have better prognosis. This could be due to the current system in Korea, where patients without a pre-existing condition are first admitted to a residential treatment center and are transferred to a hospital only after the disease progresses, and there were no differences between the groups in the multivariate analysis. For the blood test, mid-treatment results, as opposed to early blood test results, significantly differed between survivors and patients who died in the young patient group, but this difference was also not significant in the multivariate analysis, highlighting the possibility of organ failure markers. Nosocomial infection
14 and hypertension were identified as risk factors in some studies and were insignificant in others, and these factors did not significantly predict mortality in our study. Recently, there have been doubts on the validity of various criteria for identifying predictors. This is because it is difficult to identify consistent mortality predictors, other than age, in studies that include many patients in the real world, and the actual predictability of various known criteria is not high.
1819 Continued research is needed to determine significant risk factors.
All eight cases of death in the ≤ 50 years group were unvaccinated (n = 7) or incompletely vaccinated (≥ 90 days from first dose, n = 1). Although the results were statistically insignificant due to the small number of deaths and 0% complete vaccination rate among patients who died, only three individuals even in the entire young critical patient group (≤ 50 years) were fully vaccinated, and only 13.6% of the patients in the total patient population were fully vaccinated. Considering that the second-dose vaccination rate among Korean adults reached 80% as of July 3,2021 and 90.54% as of November 30, 2021
20 and that the complete vaccination rate of all confirmed patients in November was more than 80%,
21 the fact that more than 95% of the critical young adult cases and none of the patients who died were not fully vaccinated is notable. A recent press release by the Korean government showing that getting a booster shot lowers the risk of progressing to a critical case
22 can also be understood in a similar context with the high rate of non-vaccination among critical patients in our study. A clinical trial and a real-world, large-scale observational study conducted in various countries around the world reported that vaccination significantly lowers the risk.
2324 Despite the fact that usefulness and safety of vaccination have been proven in a meta-analysis
25 and systemic review,
26 there is continued controversy on the adverse events and efficacy of vaccination
27 in Korea. Our results that all cases of death in the ≤ 50 years group were non-vaccinated, and that only three out of 142 young critical patients were fully vaccinated are significant, and this data attests to the importance of vaccination to prevent critical progression and death from COVID-19.
This study has a few limitations. First, this study was a retrospective study conducted on a relatively narrow range of patients. Because we compared survivors with patients who died among those who had a critical COVID-19 infection, as opposed to the entire COVID-19 patient population, it is possible that variables that were found to be significant in other studies (vaccination, obesity) were not significant in our study. Therefore, our results cannot be generalized to the entire patient population, and follow-up studies with a broader range of patients are needed. Nevertheless, the analysis on CFR-S and risk factors for mortality in critical patients, especially in younger adults, would provide insights for management of critical patients. Furthermore, this study might represent national data because patients admitted to hospitals nationwide were included.
Second, we examined data from the fourth wave, during which the delta variant was the dominant variant. Hence, the features of the wave may differ from that involving the Omicron variant or other subsequent variants. However, the risk factors identified in our study were similar to those identified by previous studies prior to the advent of this variant, and better reflecting the delta variant surge could actually be a strength of this study. A similar type of study should be performed for the omicron surge.
Third, we defined obesity as patient with a BMI over 25, which is lower than other studies that defined obesity as a BMI over 30. This may have influenced the outcome in which BMI was not significant as a mortality risk factor. However, it should be taken into account that the BMI of Asians is generally low.
28 Also, BMI over 30 was not significant as a mortality risk factor in our study. We considered BMI over 25, considered as obesity criteria in Korea, as an analysis more suitable for the Korean situation than a BMI over 30.
Finally, there were only eight deaths in the ≤ 50 years group, which might have contributed to the lack of statistical significance for some results. Both age-related features and examination of only the Delta variant-dominant period seemed to have contributed to the results. However, we tried to complement our findings by conducting a multicenter study and by additionally analyzing the characteristics of patients who died to present information about mortality risk factors and individual patient status information. Despite these limitations, however, we believe that we have presented valuable data on the clinical outcomes and resource needs among the younger COVID-19 patient populations—a group of patients that has been documented to be relatively safe against COVID-19 and thus has sometimes been considered an exemption to vaccination and aggressive protection policies—as well as providing evidence for further research in the future.
In conclusion, the rate of obesity was high in young (≤ 50 years) critical cases, with a CFR-S of above 5% in this patient group; more than 20% did not have an underlying disease or obesity. The vaccination rate in our total patient population was significantly lower than that in the same age group in the general population. None of the patients aged 50 years and younger who died were not fully vaccinated. It should be noted that even young, healthy adults without a pre-existing condition could progress to a critical case or even death, and more attention should be paid to preventive measures, such as vaccination, targeting this population. Additional studies are needed with larger patient population.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download