3. Helal A, Shahin L, Abdelsalam M, Ibrahim M. Global effect of COVID-19 pandemic on the rate of acute coronary syndrome admissions: a comprehensive review of published literature. Open Heart. 2021; 8(1):e001645. PMID:
34083389.
4. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018; 72(18):2231–2264. PMID:
30153967.
5. Nathan AS, Raman S, Yang N, Painter I, Khatana SA, Dayoub EJ, et al. Association between 90-minute door-to-balloon time, selective exclusion of myocardial infarction cases, and access site choice: insights from the cardiac care outcomes assessment program (COAP) in Washington State. Circ Cardiovasc Interv. 2020; 13(9):e009179. PMID:
32883103.
6. Chandrasekhar J, Marley P, Allada C, McGill D, O’Connor S, Rahman M, et al. Symptom-to-balloon time is a strong predictor of adverse events following primary percutaneous coronary intervention: results from the Australian Capital Territory PCI Registry. Heart Lung Circ. 2017; 26(1):41–48. PMID:
27451348.
7. Park J, Choi KH, Lee JM, Kim HK, Hwang D, Rhee TM, et al. Prognostic implications of door-to-balloon time and onset-to-door time on mortality in patients with ST -segment-elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Heart Assoc. 2019; 8(9):e012188. PMID:
31041869.
8. Visseren FL, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021; 42(34):3227–3337. PMID:
34458905.
9. Aldujeli A, Hamadeh A, Briedis K, Tecson KM, Rutland J, Krivickas Z, et al. Delays in presentation in patients with acute myocardial infarction during the COVID-19 pandemic. Cardiol Rev. 2020; 11(6):386–391.
10. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L, Taylor DD. Excess deaths from COVID-19 and other causes, March-July 2020. JAMA. 2020; 324(15):1562–1564. PMID:
33044483.
11. Morelli N, Rota E, Terracciano C, Immovilli P, Spallazzi M, Colombi D, et al. The baffling case of ischemic stroke disappearance from the casualty department in the COVID-19 era. Eur Neurol. 2020; 83(2):213–215. PMID:
32289789.
12. Aguiar de Sousa D, Sandset EC, Elkind MS. The curious case of the missing strokes during the COVID-19 pandemic. Stroke. 2020; 51(7):1921–1923. PMID:
32466737.
13. Ruiz-Roso MB, Knott-Torcal C, Matilla-Escalante DC, Garcimartín A, Sampedro-Nuñez MA, Dávalos A, et al. COVID-19 lockdown and changes of the dietary pattern and physical activity habits in a cohort of patients with type 2 diabetes mellitus. Nutrients. 2020; 12(8):2327.
14. Navarro-Cruz AR, Kammar-García A, Mancilla-Galindo J, Quezada-Figueroa G, Tlalpa-Prisco M, Vera-López O, et al. Association of differences in dietary behaviours and lifestyle with self-reported weight gain during the COVID-19 lockdown in a university community from Chile: a cross-sectional study. Nutrients. 2021; 13(9):3213. PMID:
34579090.
15. Sorić T, Brodić I, Mertens E, Sagastume D, Dolanc I, Jonjić A, et al. Evaluation of the food choice motives before and during the COVID-19 pandemic: a cross-sectional study of 1232 adults from Croatia. Nutrients. 2021; 13(9):3165. PMID:
34579041.
16. Venter ZS, Aunan K, Chowdhury S, Lelieveld J. COVID-19 lockdowns cause global air pollution declines. Proc Natl Acad Sci U S A. 2020; 117(32):18984–18990. PMID:
32723816.
17. Tanzer-Gruener R, Li J, Eilenberg SR, Robinson AL, Presto AA. Impacts of modifiable factors on ambient air pollution: a case study of COVID-19 shutdowns. Environ Sci Technol Lett. 2020; 7(8):554–559.
18. Azuma K, Kagi N, Kim H, Hayashi M. Impact of climate and ambient air pollution on the epidemic growth during COVID-19 outbreak in Japan. Environ Res. 2020; 190:110042. PMID:
32800895.
19. Son JY, Fong KC, Heo S, Kim H, Lim CC, Bell ML. Reductions in mortality resulting from reduced air pollution levels due to COVID-19 mitigation measures. Sci Total Environ. 2020; 744:141012. PMID:
32693269.
20. Hollander JE, Carr BG. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020; 382(18):1679–1681. PMID:
32160451.
21. Portnoy J, Waller M, Elliott T. Telemedicine in the era of COVID-19. J Allergy Clin Immunol Pract. 2020; 8(5):1489–1491. PMID:
32220575.
22. Okuhara T, Okada H, Kiuchi T. Examining persuasive message type to encourage staying at home during the COVID-19 pandemic and social lockdown: a randomized controlled study in Japan. Patient Educ Couns. 2020; 103(12):2588–2593.
23. Knell G, Robertson MC, Dooley EE, Burford K, Mendez KS. Health behavior changes during COVID-19 pandemic and subsequent “Stay-at-Home” orders. Int J Environ Res Public Health. 2020; 17(17):6268.
24. Limbers CA, McCollum C, Greenwood E. Physical activity moderates the association between parenting stress and quality of life in working mothers during the COVID-19 pandemic. Ment Health Phys Act. 2020; 19:100358. PMID:
33072187.
25. Gluckman TJ, Wilson MA, Chiu ST, Penny BW, Chepuri VB, Waggoner JW, et al. Case rates, treatment approaches, and outcomes in acute myocardial infarction during the coronavirus disease 2019 pandemic. JAMA Cardiol. 2020; 5(12):1419–1424. PMID:
32766756.
26. Marotta M, Gorini F, Parlanti A, Chatzianagnostou K, Mazzone A, Berti S, et al. Fear of COVID-19 in patients with acute myocardial infarction. Int J Environ Res Public Health. 2021; 18(18):9847. PMID:
34574770.
27. Ciofani JL, Han D, Allahwala UK, Asrress KN, Bhindi R. Internet search volume for chest pain during the COVID-19 pandemic. Am Heart J. 2021; 231:157–159. PMID:
33010246.
28. Baldi E, Sechi GM, Mare C, Canevari F, Brancaglione A, Primi R, et al. Out-of-hospital cardiac arrest during the COVID-19 outbreak in Italy. N Engl J Med. 2020; 383(5):496–498. PMID:
32348640.
29. Bhatt AS, Moscone A, McElrath EE, Varshney AS, Claggett BL, Bhatt DL, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020; 76(3):280–288. PMID:
32470516.
30. Mahmud E, Dauerman HL, Welt FG, Messenger JC, Rao SV, Grines C, et al. Management of acute myocardial infarction during the COVID-19 pandemic: a position statement from the Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology (ACC), and the American College of Emergency Physicians (ACEP). J Am Coll Cardiol. 2020; 76(11):1375–1384. PMID:
32330544.
31. Primessnig U, Pieske BM, Sherif M. Increased mortality and worse cardiac outcome of acute myocardial infarction during the early COVID-19 pandemic. ESC Heart Fail. 2021; 8(1):333–343. PMID:
33283476.
32. Park DW, Yang Y. Delay, death, and heterogeneity of primary PCI during the COVID-19 pandemic: an international perspective. J Am Coll Cardiol. 2020; 76(20):2331–2333. PMID:
33183507.
33. Kitahara S, Fujino M, Honda S, Asaumi Y, Kataoka Y, Otsuka F, et al. COVID-19 pandemic is associated with mechanical complications in patients with ST-elevation myocardial infarction. Open Heart. 2021; 8(1):e001497. PMID:
33547221.
34. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020; 41(19):1852–1853. PMID:
32297932.
35. Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical Renin-Angiotensin system in kidney physiology. Compr Physiol. 2014; 4(3):1201–1228. PMID:
24944035.
36. Li G, He X, Zhang L, Ran Q, Wang J, Xiong A, et al. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J Autoimmun. 2020; 112:102463. PMID:
32303424.
37. Rico-Mesa JS, White A, Anderson AS. Outcomes in patients with COVID-19 infection taking ACEI/ARB. Curr Cardiol Rep. 2020; 22(5):31. PMID:
32291526.
38. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8(5):475–481. PMID:
32105632.
39. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382(18):1708–1720. PMID:
32109013.
40. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020; 75(7):1730–1741. PMID:
32077115.
41. Hippisley-Cox J, Young D, Coupland C, Channon KM, Tan PS, Harrison DA, et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020; 106(19):1503–1511. PMID:
32737124.
42. Flacco ME, Acuti Martellucci C, Bravi F, Parruti G, Cappadona R, Mascitelli A, et al. Treatment with ACE inhibitors or ARBs and risk of severe/lethal COVID-19: a meta-analysis. Heart. 2020; 106(19):1519–1524. PMID:
32611676.
43. Rossi GP, Sanga V, Barton M. Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients. Elife. 2020; 9:e57278. PMID:
32250244.
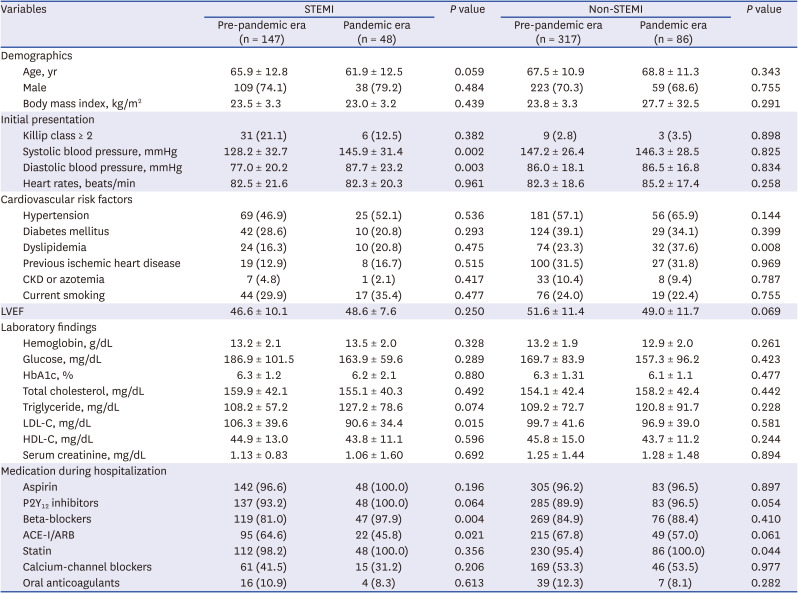
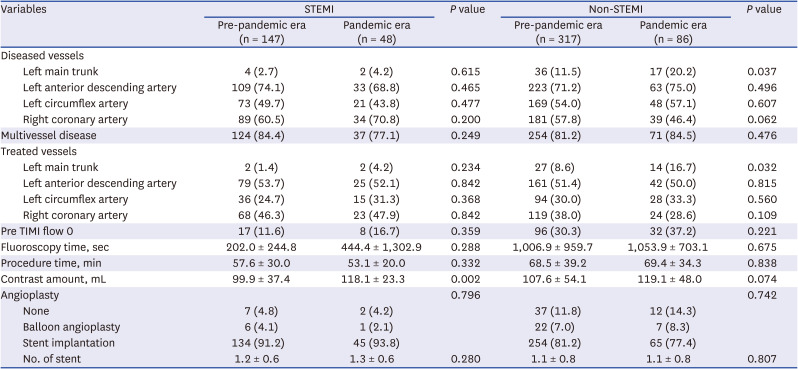
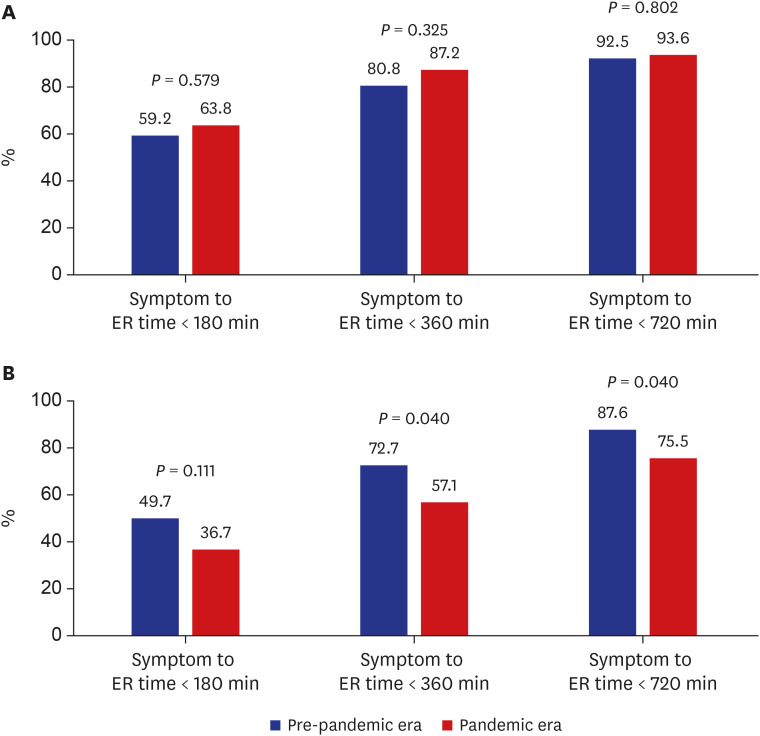
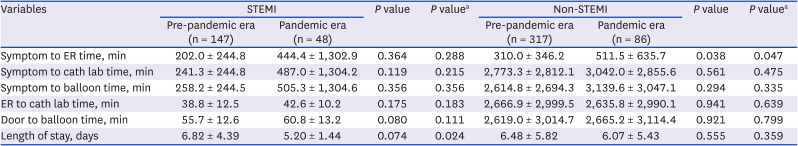




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download