1. Hall JE. Guyton and Hall Textbook of Medical Physiology. Amsterdam: Elsevier Health Sciences;2010.
2. He FJ, Li J, Macgregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2013; CD004937. PMID:
23633321.
3. World Health Organization (WHO). Guideline: Sodium Intake for Adults and Children. Geneva: WHO;2012.
4. Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013; 346:f1326. PMID:
23558163.
5. Graudal N, Jürgens G, Baslund B, Alderman MH. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: a meta-analysis. Am J Hypertens. 2014; 27:1129–1137. PMID:
24651634.
6. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018; 71:e127–e248. PMID:
29146535.
7. Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014; 371:624–634. PMID:
25119608.
8. National Academies of Sciences, Engineering, and Medicine. Dietary Reference Intakes for Sodium and Potassium. Washington, D.C.: The National Academies Press;2019.
9. Ministry of Health and Welfare (KR). Dietary Reference Intakes for Koreans 2015. Sejong: Ministry of Health and Welfare;2015.
10. Newberry SJ, Anderson CA, Chen C, Fu Z, Tang A, Zhao N, Booth M, Marks J, Hollands S, Motala A, et al. Sodium and potassium intake: effects on chronic disease outcomes and risks [Internet]. Rockville (MD): Agency for Healthcare Research and Quality;2018. cited 2021 October 5. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK519328/.
11. National Academies of Sciences, Engineering, and Medicine. Guiding Principles for Developing Dietary Reference Intakes Based on Chronic Disease. Washington, D.C.: The National Academies Press;2017.
12. Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, D.C.: The National Academies Press;2006.
13. Korea Centers for Disease Control and Prevention (KCDC). Korea Health Statistics 2017: Korea National Health and Nutrition Examination Survey (KNHANES VII-2). Cheongju: KCDC;2018.
14. Craddick SR, Elmer PJ, Obarzanek E, Vollmer WM, Svetkey LP, Swain MC. The DASH diet and blood pressure. Curr Atheroscler Rep. 2003; 5:484–491. PMID:
14525682.
15. Sharma AM, Arntz HR, Kribben A, Schattenfroh S, Distler A. Dietary sodium restriction: adverse effect on plasma lipids. Klin Wochenschr. 1990; 68:664–668. PMID:
2381134.
16. Ruppert M, Diehl J, Kolloch R, Overlack A, Kraft K, Göbel B, Hittel N, Stumpe KO. Short-term dietary sodium restriction increases serum lipids and insulin in salt-sensitive and salt-resistant normotensive adults. Klin Wochenschr. 1991; 69(Suppl 25):51–57. PMID:
1921253.
17. Grey A, Braatvedt G, Holdaway I. Moderate dietary salt restriction does not alter insulin resistance or serum lipids in normal men. Am J Hypertens. 1996; 9:317–322. PMID:
8722434.
18. Allsopp AJ, Sutherland R, Wood P, Wootton SA. The effect of sodium balance on sweat sodium secretion and plasma aldosterone concentration. Eur J Appl Physiol Occup Physiol. 1998; 78:516–521. PMID:
9840406.
19. Atkinson SA, Koletzko B. Determining life-stage groups and extrapolating nutrient intake values (NIVs). Food Nutr Bull. 2007; 28:S61–S76. PMID:
17521120.
20. Jung WJ, Park SM, Park JM, Rhee H, Kim IY, Lee DW, Lee SB, Seong EY, Kwak IS, Song SH. Severe hypernatremia caused by acute exogenous salt intake combined with primary hypothyroidism. Electrolyte Blood Press. 2016; 14:27–30. PMID:
28275385.
21. Metheny NA, Krieger MM. Salt toxicity: a systematic review and case reports. J Emerg Nurs. 2020; 46:428–439. PMID:
32340735.
22. Sakamoto A, Hoshino T, Boku K, Hiraya D, Inoue Y. Fatal acute hypernatremia resulting from a massive intake of seasoning soy sauce. Acute Med Surg. 2020; 7:e555. PMID:
32832094.
23. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons-Morton DG, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001; 344:3–10. PMID:
11136953.
24. Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH Jr, Kostis JB, Kumanyika S, Lacy CR, Johnson KC, Folmar S, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). JAMA. 1998; 279:839–846. PMID:
9515998.
25. Amer M, Woodward M, Appel LJ. Effects of dietary sodium and the DASH diet on the occurrence of headaches: results from randomised multicentre DASH-Sodium clinical trial. BMJ Open. 2014; 4:e006671.
26. Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, Engell RE, Lim SS, Danaei G, Mozaffarian D, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013; 3:e003733.
27. Elliott P, Brown I. Sodium Intakes Around the World. Geneva: World Health Organization;2007.
28. Korea Disease Control and Prevention Agency. Korea Health Statistics 2019: Korea National Health and Nutritional Examination Survey (KNHANES VIII-1). Cheongju: Korea Disease Control and Prevention Agency;2020.
29. Rural Development Administration (KR). Food Composition Database 9.1. Wanju: Rural Development Administration;2019.
30. Mente A, O’Donnell M, Rangarajan S, Dagenais G, Lear S, McQueen M, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. 2016; 388:465–475. PMID:
27216139.
31. Teramoto T, Kawamori R, Miyazaki S, Teramukai S. OMEGA Study Group. Sodium intake in men and potassium intake in women determine the prevalence of metabolic syndrome in Japanese hypertensive patients: OMEGA Study. Hypertens Res. 2011; 34:957–962. PMID:
21654751.
32. Geleijnse JM, Witteman JC, Stijnen T, Kloos MW, Hofman A, Grobbee DE. Sodium and potassium intake and risk of cardiovascular events and all-cause mortality: the Rotterdam Study. Eur J Epidemiol. 2007; 22:763–770. PMID:
17902026.
33. Yang Q, Liu T, Kuklina EV, Flanders WD, Hong Y, Gillespie C, Chang MH, Gwinn M, Dowling N, Khoury MJ, et al. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2011; 171:1183–1191. PMID:
21747015.
34. Perez V, Chang ET. Sodium-to-potassium ratio and blood pressure, hypertension, and related factors. Adv Nutr. 2014; 5:712–741. PMID:
25398734.
35. Du S, Batis C, Wang H, Zhang B, Zhang J, Popkin BM, Popkin BM. Understanding the patterns and trends of sodium intake, potassium intake, and sodium to potassium ratio and their effect on hypertension in China. Am J Clin Nutr. 2014; 99:334–343. PMID:
24257724.
36. Willey J, Gardener H, Cespedes S, Cheung YK, Sacco RL, Elkind MS. Dietary sodium to potassium ratio and risk of stroke in a multiethnic urban population: the Northern Manhattan Study. Stroke. 2017; 48:2979–2983. PMID:
29018136.
37. Korea Disease Control and Prevention Agency. The Seventh Korea National Health and Nutrition Examination Survey (KNHANES VII-1). Cheongju: Korea Disease Control and Prevention Agency;2019.
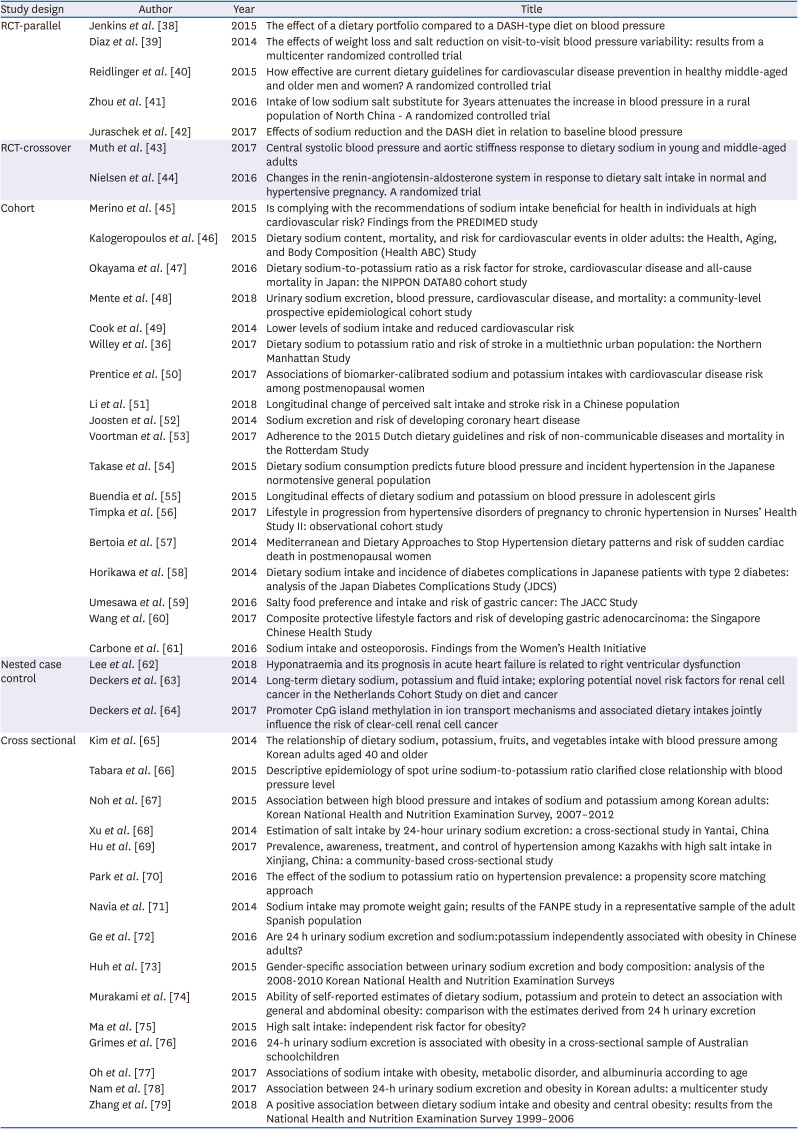
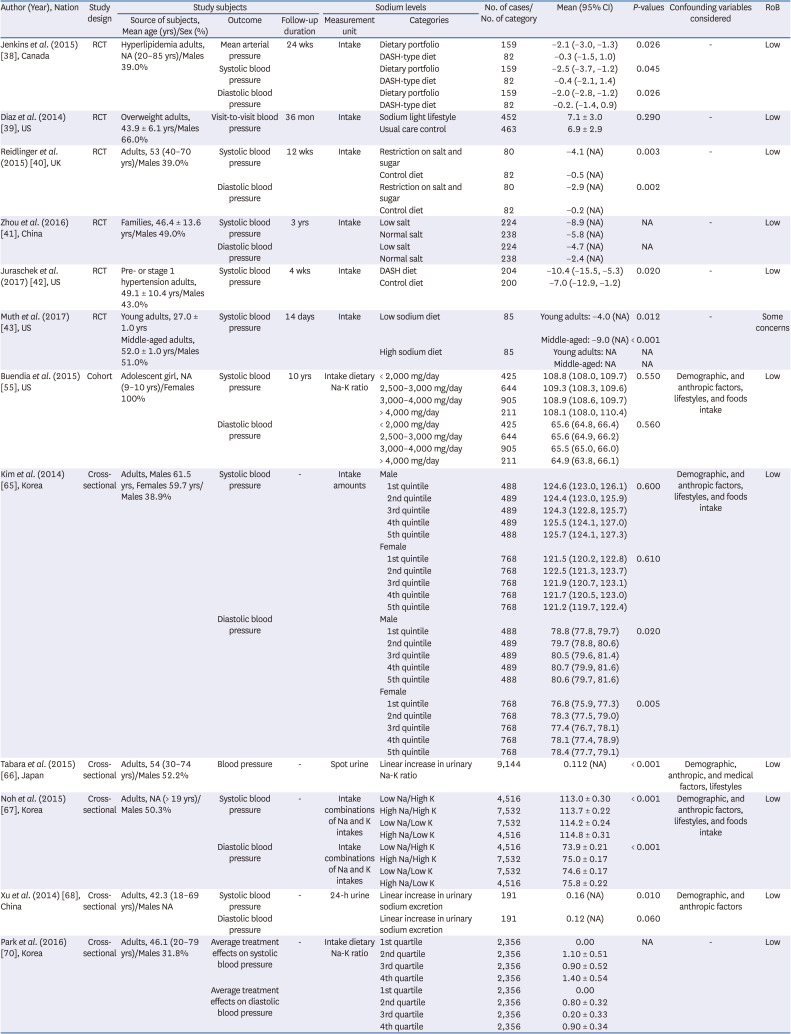
38. Jenkins DJ, Jones PJ, Frohlich J, Lamarche B, Ireland C, Nishi SK, Srichaikul K, Galange P, Pellini C, Faulkner D, et al. The effect of a dietary portfolio compared to a DASH-type diet on blood pressure. Nutr Metab Cardiovasc Dis. 2015; 25:1132–1139. PMID:
26552742.
39. Diaz KM, Muntner P, Levitan EB, Brown MD, Babbitt DM, Shimbo D. The effects of weight loss and salt reduction on visit-to-visit blood pressure variability: results from a multicenter randomized controlled trial. J Hypertens. 2014; 32:840–848. PMID:
24366034.
40. Reidlinger DP, Darzi J, Hall WL, Seed PT, Chowienczyk PJ, Sanders TA. Cardiovascular disease risk REduction Study (CRESSIDA) investigators. How effective are current dietary guidelines for cardiovascular disease prevention in healthy middle-aged and older men and women? A randomized controlled trial. Am J Clin Nutr. 2015; 101:922–930. PMID:
25787998.
41. Zhou B, Webster J, Fu LY, Wang HL, Wu XM, Wang WL, Shi JP. Intake of low sodium salt substitute for 3years attenuates the increase in blood pressure in a rural population of North China - A randomized controlled trial. Int J Cardiol. 2016; 215:377–382. PMID:
27128565.
42. Juraschek SP, Miller ER 3rd, Weaver CM, Appel LJ. Effects of sodium reduction and the DASH diet in relation to baseline blood pressure. J Am Coll Cardiol. 2017; 70:2841–2848. PMID:
29141784.
43. Muth BJ, Brian MS, Chirinos JA, Lennon SL, Farquhar WB, Edwards DG. Central systolic blood pressure and aortic stiffness response to dietary sodium in young and middle-aged adults. J Am Soc Hypertens. 2017; 11:627–634. PMID:
28830669.
44. Nielsen LH, Ovesen P, Hansen MR, Brantlov S, Jespersen B, Bie P, Jensen BL. Changes in the renin-angiotensin-aldosterone system in response to dietary salt intake in normal and hypertensive pregnancy. A randomized trial. J Am Soc Hypertens. 2016; 10:881–890.e4. PMID:
27836073.
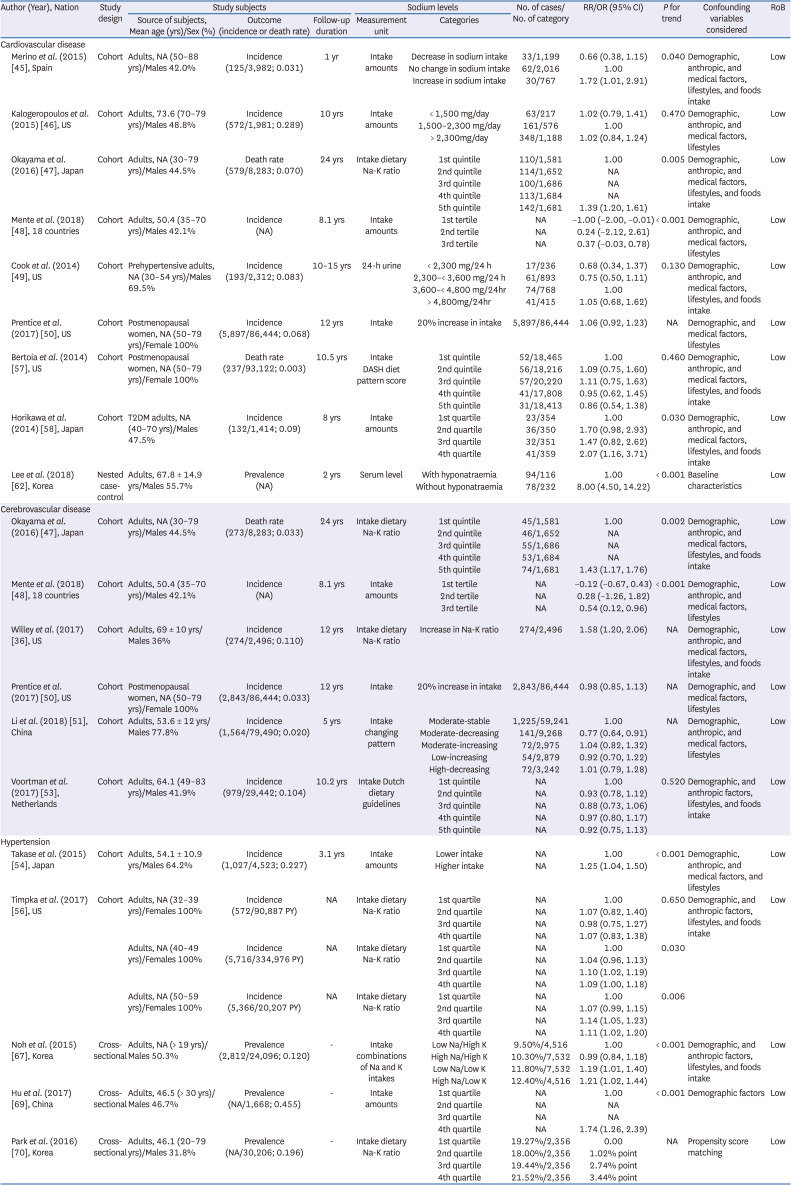
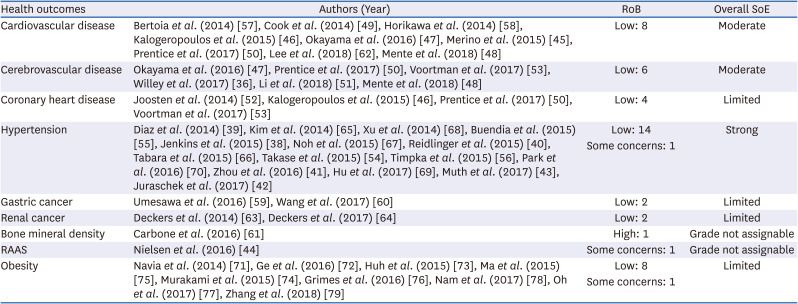
45. Merino J, Guasch-Ferré M, Martínez-González MA, Corella D, Estruch R, Fitó M, Ros E, Arós F, Bulló M, Gómez-Gracia E, et al. Is complying with the recommendations of sodium intake beneficial for health in individuals at high cardiovascular risk? Findings from the PREDIMED study. Am J Clin Nutr. 2015; 101:440–448. PMID:
25733627.
46. Kalogeropoulos AP, Georgiopoulou VV, Murphy RA, Newman AB, Bauer DC, Harris TB, Yang Z, Applegate WB, Kritchevsky SB. Dietary sodium content, mortality, and risk for cardiovascular events in older adults: the Health, Aging, and Body Composition (Health ABC) Study. JAMA Intern Med. 2015; 175:410–419. PMID:
25599120.
47. Okayama A, Okuda N, Miura K, Okamura T, Hayakawa T, Akasaka H, Ohnishi H, Saitoh S, Arai Y, Kiyohara Y, et al. Dietary sodium-to-potassium ratio as a risk factor for stroke, cardiovascular disease and all-cause mortality in Japan: the NIPPON DATA80 cohort study. BMJ Open. 2016; 6:e011632.
48. Mente A, O’Donnell M, Rangarajan S, McQueen M, Dagenais G, Wielgosz A, Lear S, Ah ST, Wei L, Diaz R, et al. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: a community-level prospective epidemiological cohort study. Lancet. 2018; 392:496–506. PMID:
30129465.
49. Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014; 129:981–989. PMID:
24415713.
50. Prentice RL, Huang Y, Neuhouser ML, Manson JE, Mossavar-Rahmani Y, Thomas F, Tinker LF, Allison M, Johnson KC, Wassertheil-Smoller S, et al. Associations of biomarker-calibrated sodium and potassium intakes with cardiovascular disease risk among postmenopausal women. Am J Epidemiol. 2017; 186:1035–1043. PMID:
28633342.
51. Li Y, Huang Z, Jin C, Xing A, Liu Y, Huangfu C, Lichtenstein AH, Tucker KL, Wu S, Gao X. Longitudinal change of perceived salt intake and stroke risk in a chinese population. Stroke. 2018; 49:1332–1339. PMID:
29739913.
52. Joosten MM, Gansevoort RT, Mukamal KJ, Lambers Heerspink HJ, Geleijnse JM, Feskens EJ, Navis G, Bakker SJ. PREVEND Study Group. Sodium excretion and risk of developing coronary heart disease. Circulation. 2014; 129:1121–1128. PMID:
24425751.
53. Voortman T, Kiefte-de Jong JC, Ikram MA, Stricker BH, van Rooij FJ, Lahousse L, Tiemeier H, Brusselle GG, Franco OH, Schoufour JD. Adherence to the 2015 Dutch dietary guidelines and risk of non-communicable diseases and mortality in the Rotterdam Study. Eur J Epidemiol. 2017; 32:993–1005. PMID:
28825166.
54. Takase H, Sugiura T, Kimura G, Ohte N, Dohi Y.. Dietary sodium consumption predicts future blood pressure and incident hypertension in the Japanese normotensive general population. J Am Heart Assoc. 2015; 4:e001959. PMID:
26224048.
55. Buendia JR, Bradlee ML, Daniels SR, Singer MR, Moore LL. Longitudinal effects of dietary sodium and potassium on blood pressure in adolescent girls. JAMA Pediatr. 2015; 169:560–568. PMID:
25915457.
56. Timpka S, Stuart JJ, Tanz LJ, Rimm EB, Franks PW, Rich-Edwards JW. Lifestyle in progression from hypertensive disorders of pregnancy to chronic hypertension in Nurses’ Health Study II: observational cohort study. BMJ. 2017; 358:j3024. PMID:
28701338.
57. Bertoia ML, Triche EW, Michaud DS, Baylin A, Hogan JW, Neuhouser ML, Tinker LF, Van Horn L, Waring ME, Li W, et al. Mediterranean and Dietary Approaches to Stop Hypertension dietary patterns and risk of sudden cardiac death in postmenopausal women. Am J Clin Nutr. 2014; 99:344–351. PMID:
24351877.
58. Horikawa C, Yoshimura Y, Kamada C, Tanaka S, Tanaka S, Hanyu O, Araki A, Ito H, Tanaka A, Ohashi Y, et al. Dietary sodium intake and incidence of diabetes complications in Japanese patients with type 2 diabetes: analysis of the Japan Diabetes Complications Study (JDCS). J Clin Endocrinol Metab. 2014; 99:3635–3643. PMID:
25050990.
59. Umesawa M, Iso H, Fujino Y, Kikuchi S, Tamakoshi A. JACC Study Group. Salty food preference and intake and risk of gastric cancer: the JACC study. J Epidemiol. 2016; 26:92–97. PMID:
26477994.
60. Wang Z, Koh WP, Jin A, Wang R, Yuan JM. Composite protective lifestyle factors and risk of developing gastric adenocarcinoma: the Singapore Chinese Health Study. Br J Cancer. 2017; 116:679–687. PMID:
28125822.
61. Carbone L, Johnson KC, Huang Y, Pettinger M, Thomas F, Cauley J, Crandall C, Tinker L, LeBoff MS, Wactawski-Wende J, et al. Sodium intake and osteoporosis. J Clin Endocrinol Metab. 2016; 101:1414–1421. PMID:
26863423.
62. Lee H, Lee SE, Park CS, Park JJ, Lee GY, Kim MS, Choi JO, Cho HJ, Lee HY, Choi DJ, et al. Hyponatraemia and its prognosis in acute heart failure is related to right ventricular dysfunction. Heart. 2018; 104:1670–1677. PMID:
29079633.
63. Deckers IA, van den Brandt PA, van Engeland M, Soetekouw PM, Baldewijns MM, Goldbohm RA, Schouten LJ. Long-term dietary sodium, potassium and fluid intake; exploring potential novel risk factors for renal cell cancer in the Netherlands Cohort Study on diet and cancer. Br J Cancer. 2014; 110:797–801. PMID:
24327014.
64. Deckers IA, van Engeland M, van den Brandt PA, Van Neste L, Soetekouw PM, Aarts MJ, Baldewijns MM, Keszei AP, Schouten LJ. Promoter CpG island methylation in ion transport mechanisms and associated dietary intakes jointly influence the risk of clear-cell renal cell cancer. Int J Epidemiol. 2017; 46:622–631. PMID:
27789672.
65. Kim MK, Kim K, Shin MH, Shin DH, Lee YH, Chun BY, Choi BY. The relationship of dietary sodium, potassium, fruits, and vegetables intake with blood pressure among Korean adults aged 40 and older. Nutr Res Pract. 2014; 8:453–462. PMID:
25110567.
66. Tabara Y, Takahashi Y, Kumagai K, Setoh K, Kawaguchi T, Takahashi M, Muraoka Y, Tsujikawa A, Gotoh N, Terao C, et al. Descriptive epidemiology of spot urine sodium-to-potassium ratio clarified close relationship with blood pressure level: the Nagahama study. J Hypertens. 2015; 33:2407–2413. PMID:
26378682.
67. Noh HM, Park SY, Lee HS, Oh HY, Paek YJ, Song HJ, Park KH. Association between High blood pressure and intakes of sodium and potassium among Korean adults: Korean National Health and Nutrition Examination Survey, 2007-2012. J Acad Nutr Diet. 2015; 115:1950–1957. PMID:
26129945.
68. Xu J, Wang M, Chen Y, Zhen B, Li J, Luan W, Ning F, Liu H, Ma J, Ma G. Estimation of salt intake by 24-hour urinary sodium excretion: a cross-sectional study in Yantai, China. BMC Public Health. 2014; 14:136. PMID:
24507470.
69. Hu Y, Wang Z, Wang Y, Wang L, Han W, Tang Y, Xue F, Hou L, Liang S, Zhang B, et al. Prevalence, awareness, treatment, and control of hypertension among Kazakhs with high salt intake in Xinjiang, China: a community-based cross-sectional study. Sci Rep. 2017; 7:45547. PMID:
28358015.
70. Park J, Kwock CK, Yang YJ. The effect of the sodium to potassium ratio on hypertension prevalence: a propensity score matching approach. Nutrients. 2016; 8:482.
71. Navia B, Aparicio A, Perea JM, Pérez-Farinós N, Villar-Villalba C, Labrado E, Ortega RM. Sodium intake may promote weight gain; results of the FANPE study in a representative sample of the adult Spanish population. Nutr Hosp. 2014; 29:1283–1289. PMID:
24972464.
72. Ge Z, Zhang J, Chen X, Yan L, Guo X, Lu Z, Xu A, Ma J. Are 24 h urinary sodium excretion and sodium:potassium independently associated with obesity in Chinese adults? Public Health Nutr. 2016; 19:1074–1080. PMID:
26228639.
73. Huh JH, Lim JS, Lee MY, Chung CH, Shin JY. Gender-specific association between urinary sodium excretion and body composition: analysis of the 2008-2010 Korean National Health and Nutrition Examination Surveys. Metabolism. 2015; 64:837–844. PMID:
25873364.
74. Murakami K, Livingstone MB, Sasaki S, Uenishi K. Japan Dietetic Students’ Study for Nutrition and Biomarkers Group. Ability of self-reported estimates of dietary sodium, potassium and protein to detect an association with general and abdominal obesity: comparison with the estimates derived from 24 h urinary excretion. Br J Nutr. 2015; 113:1308–1318. PMID:
25782331.
75. Ma Y, He FJ, MacGregor GA. High salt intake: independent risk factor for obesity? Hypertension. 2015; 66:843–849. PMID:
26238447.
76. Grimes CA, Riddell LJ, Campbell KJ, He FJ, Nowson CA. 24-h urinary sodium excretion is associated with obesity in a cross-sectional sample of Australian schoolchildren. Br J Nutr. 2016; 115:1071–1079. PMID:
26810972.
77. Oh SW, Koo HS, Han KH, Han SY, Chin HJ. Associations of sodium intake with obesity, metabolic disorder, and albuminuria according to age. PLoS One. 2017; 12:e0188770. PMID:
29244825.
78. Nam GE, Kim SM, Choi MK, Heo YR, Hyun TS, Lyu ES, Oh SY, Park HR, Ro HK, Han K, et al. Association between 24-h urinary sodium excretion and obesity in Korean adults: a multicenter study. Nutrition. 2017; 41:113–119. PMID:
28760420.
79. Zhang X, Wang J, Li J, Yu Y, Song Y. A positive association between dietary sodium intake and obesity and central obesity: results from the National Health and Nutrition Examination Survey 1999-2006. Nutr Res. 2018; 55:33–44. PMID:
29914626.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download