1. Jones PJH, Papamandjaris AA. Lipids: cellular metabolism. Bowman BA, Russell RM, editors. Present Knowledge in Nutrition. Washington, D.C.: International Life Sciences Institute;2001.
2. de Roos B, Mavrommatis Y, Brouwer IA. Long-chain n-3 polyunsaturated fatty acids: new insights into mechanisms relating to inflammation and coronary heart disease. Br J Pharmacol. 2009; 158:413–428. PMID:
19422375.
3. Park Y, Watkins BA. Endocannabinoids and aging-inflammation, neuroplasticity, mood and pain. Vitam Horm. 2021; 115:129–172. PMID:
33706946.
4. Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, D.C.: The National Academies Press;2005.
5. Das UN. Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J. 2006; 1:420–439. PMID:
16892270.
6. Burdge GC, Finnegan YE, Minihane AM, Williams CM, Wootton SA. Effect of altered dietary n-3 fatty acid intake upon plasma lipid fatty acid composition, conversion of [13C]alpha-linolenic acid to longer-chain fatty acids and partitioning towards beta-oxidation in older men. Br J Nutr. 2003; 90:311–321. PMID:
12908891.
7. Balk EM, Adams GP, Langberg V, Halladay C, Chung M, Lin L, Robertson S, Yip A, Steele D, Smith BT, et al. Omega-3 fatty acids and cardiovascular disease: an updated systematic review. Evid Rep Technol Assess (Full Rep). 2016; 1–1252.
8. Ministry of Health and Welfare, The Korean Nutrition Society. Dietary reference intakes for Koreans 2020. Sejong: Ministry of Health and Welfare;2020.
9. Institute of Medicine. Dietary reference intakes: the essential guide to nutrient requirements. Washington, D.C.: The National Academies Press;2010.
10. Kris-Etherton PM, Innis S. Ammerican Dietetic Association. Dietitians of Canada. Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids. J Am Diet Assoc. 2007; 107:1599–1611. PMID:
17936958.
11. Food and Agriculture Organization of United Nations. FAO food and nutrition paper. Fats and fatty acids in human nutrition. Geneva: WHO;2008. p. 1–166.
12. Moon SJ, Lee MJ, Kim JH, Kang JS, Ahn HS, Song SW, Choi MH. A longitudinal study of the total nitrogen, total lipid, and lactose contents in human milk and energy intake of breast-fed infants. Korean J Nutr. 1992; 25:233–247.
13. Lim HS, Lee JA, Huh YR, Lee JI. Intakes of energy, protein, lipid and lactose in Korean breast-fed and formula-fed infants. Korean J Nutr. 1993; 26:325–337.
14. Bai HS, Lee DH, Ahn HS. Nutrient intakes of infants according to feeding pattern at 2month age. Korean J Nutr. 1996; 29:77–88.
15. Choi KS, Kim ES. A longitudinal study on energy, protein, lipid and lactose intakes of breast-fed infants of lacto-ovo-vegetarian. Korean J Nutr. 1997; 30:512–519.
16. Ahn HS, Jeong JY. Ecological studies of maternal-infant nutrition and feeding in urban low income areas III. Infant’s nutrient intakes and growth pattern. Korean J Community Nutr. 1998; 3:174–189.
17. Kim ES, Lee JS. A longitudinal study on energy, protein, fat and lactose intake of breast-fed infants. Korean J Nutr. 2002; 35:771–778.
18. Ahn HS, Um SS. Dietary intakes of infants and young children in Seoul area. J Korean Soc Matern Child Health. 2003; 7:179–191.
19. Duan B, Shin JA, Qin Y, Kwon JI, Lee KT. A study on the relationship of fat content in human milk on carotenoids content and fatty acid compositions in Korea. Nutrients. 2019; 11:2072.
20. European Food Safety Authority. Dietary reference values for nutrients summary report. Parma: EFSA Supporting Publications;2017. p. 14.
21. Ervin RB, Wright JD, Wang CY, Kennedy-Stephenson J. Dietary intake of fats and fatty acids for the United States population: 1999–2000. Adv Data. 2004; 1–6.
22. Stark KD, Van Elswyk ME, Higgins MR, Weatherford CA, Salem N Jr. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog Lipid Res. 2016; 63:132–152. PMID:
27216485.
23. Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, Hu H, Gillman MW. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am J Epidemiol. 2008; 167:1171–1181. PMID:
18353804.
24. Oken E, Rifas-Shiman SL, Amarasiriwardena C, Jayawardene I, Bellinger DC, Hibbeln JR, Wright RO, Gillman MW. Maternal prenatal fish consumption and cognition in mid childhood: mercury, fatty acids, and selenium. Neurotoxicol Teratol. 2016; 57:71–78. PMID:
27381635.
25. Mozaffarian D, Shi P, Morris JS, Spiegelman D, Grandjean P, Siscovick DS, Willett WC, Rimm EB. Mercury exposure and risk of cardiovascular disease in two U.S. cohorts. N Engl J Med. 2011; 364:1116–1125. PMID:
21428767.
26. Mozaffarian D, Shi P, Morris JS, Grandjean P, Siscovick DS, Spiegelman D, Willett WC, Rimm EB, Curhan GC, Forman JP. Mercury exposure and risk of hypertension in US men and women in 2 prospective cohorts. Hypertension. 2012; 60:645–652. PMID:
22868395.
27. Virtanen JK, Laukkanen JA, Mursu J, Voutilainen S, Tuomainen TP. Serum long-chain n-3 polyunsaturated fatty acids, mercury, and risk of sudden cardiac death in men: a prospective population-based study. PLoS One. 2012; 7:e41046. PMID:
22815906.
28. Ministry of Health, Labour and Welfare of Japan. Overview of dietary reference intakes for Japanese. Tokyo: Ministry of Health, Labour and Welfare of Japan;2020.
29. Harris WS, Park Y, Isley WL. Cardiovascular disease and long-chain omega-3 fatty acids. Curr Opin Lipidol. 2003; 14:9–14. PMID:
12544655.
30. Kang JX, Leaf A. Prevention of fatal cardiac arrhythmias by polyunsaturated fatty acids. Am J Clin Nutr. 2000; 71:202S–2027S. PMID:
10617972.
31. Chiuve SE, Rimm EB, Sandhu RK, Bernstein AM, Rexrode KM, Manson JE, Willett WC, Albert CM. Dietary fat quality and risk of sudden cardiac death in women. Am J Clin Nutr. 2012; 96:498–507. PMID:
22854398.
32. Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995; 274:1363–1367. PMID:
7563561.
33. Albert CM, Hennekens CH, O'Donnell CJ, Ajani UA, Carey VJ, Willett WC, Ruskin JN, Manson JE. Fish consumption and risk of sudden cardiac death. JAMA. 1998; 279:23–28. PMID:
9424039.
34. Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, Rimm EB. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 2005; 111:157–164. PMID:
15630029.
35. Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y, Kokubo Y, Tsugane S. JPHC Study Group. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation. 2006; 113:195–202. PMID:
16401768.
36. Yamagishi K, Iso H, Date C, Fukui M, Wakai K, Kikuchi S, Inaba Y, Tanabe N, Tamakoshi A. Japan Collaborative Cohort Study for Evaluation of Cancer Risk Study Group. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol. 2008; 52:988–996. PMID:
18786479.
37. Shuppan D. The national nutrition survey in Japan, 2001. Health and Nutrition Information Research Committee. Tokyo: Ministry of Health, Labour and Welfare;2003.
38. Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 1989; 2:757–761. PMID:
2571009.
39. Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002; 105:1897–1903. PMID:
11997274.
40. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007; 369:1090–1098. PMID:
17398308.
41. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019; 380:11–22. PMID:
30415628.
42. Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020; 324:2268–2280. PMID:
33190147.
43. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019; 380:23–32. PMID:
30415637.
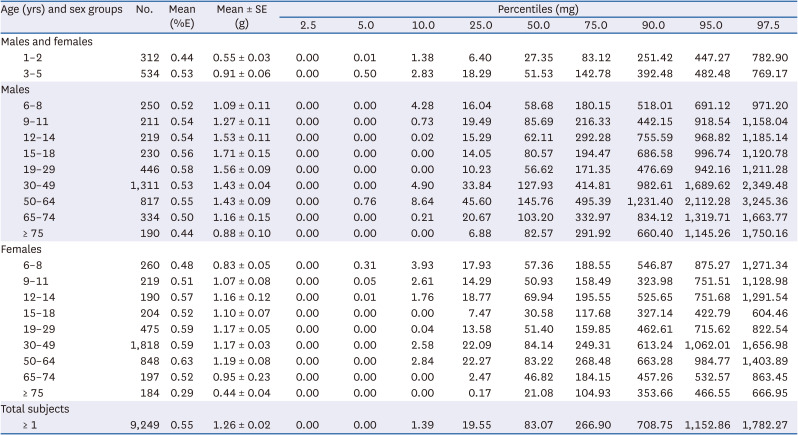
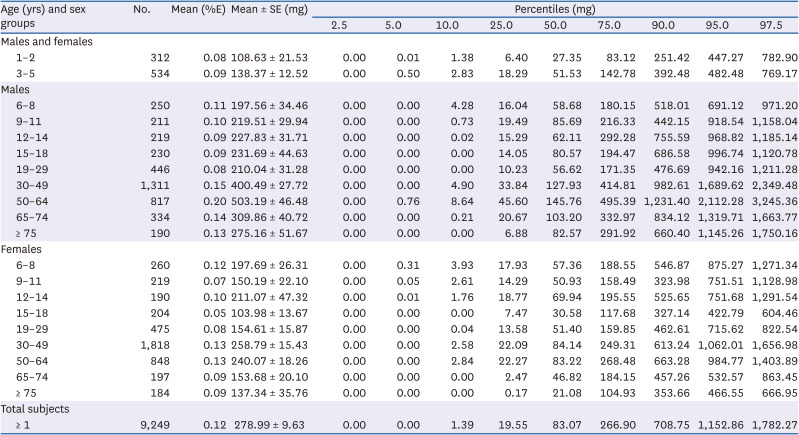
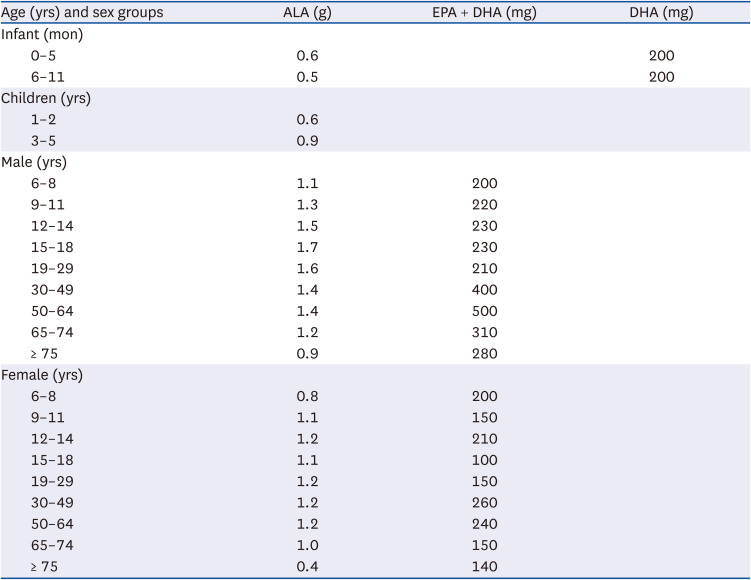
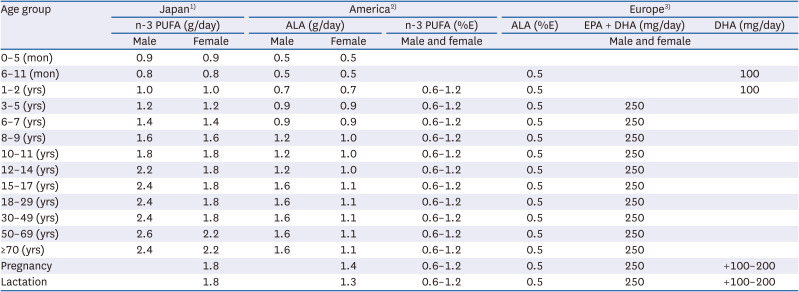




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download