1. World Health Organization/Food and Agriculture Organization of the United Nations/United Nations University (WHO/FAO/UNU). Protein and Amino Acid Requirements in Human Nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation. Geneva: World Health Organization;2007.
2. Ministry of Health and Welfare, The Korean Nutrition Society. 2020 Dietary Reference Intakes for Koreans: Energy and Macronutrients. Seoul: Ministry of Health and Welfare, The Korean Nutrition Society;2020.
3. Kim JG, Kim JS, Kim JG. Trends of food supply and nutrient intake in South Korea over the past 30 years. Curr Res Nutr Food Sci. 2019; 7:85–95.
4. Yun S, Kim HJ, Oh K. Trends in energy intake among Korean adults, 1998-2015: results from the Korea National Health and Nutrition Examination Survey. Nutr Res Pract. 2017; 11:147–154. PMID:
28386388.
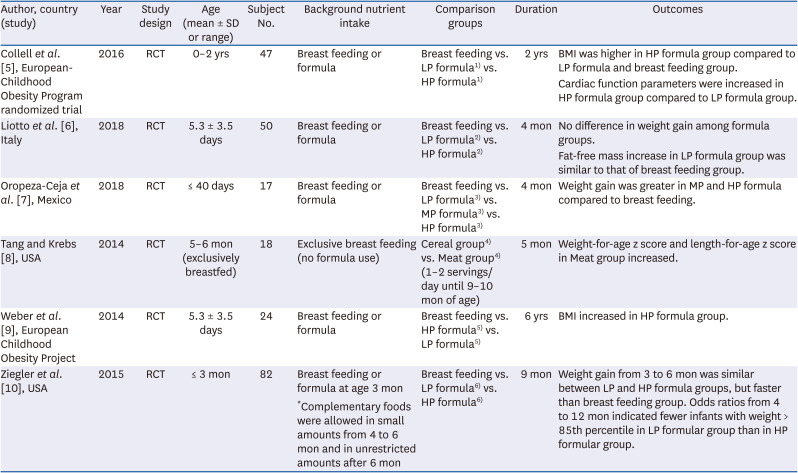
5. Collell R, Closa-Monasterolo R, Ferré N, Luque V, Koletzko B, Grote V, Janas R, Verduci E, Escribano J. Higher protein intake increases cardiac function parameters in healthy children: metabolic programming by infant nutrition-secondary analysis from a clinical trial. Pediatr Res. 2016; 79:880–888. PMID:
26882370.
6. Liotto N, Orsi A, Menis C, Piemontese P, Morlacchi L, Condello CC, Giannì ML, Roggero P, Mosca F. Clinical evaluation of two different protein content formulas fed to full-term healthy infants: a randomized controlled trial. BMC Pediatr. 2018; 18:59. PMID:
29439736.
7. Oropeza-Ceja LG, Rosado JL, Ronquillo D, García OP, Caamaño MDC, García-Ugalde C, Viveros-Contreras R, Duarte-Vázquez MÁ. Lower protein intake supports normal growth of full-term infants fed formula: a randomized controlled trial. Nutrients. 2018; 10:10.
8. Tang M, Krebs NF. High protein intake from meat as complementary food increases growth but not adiposity in breastfed infants: a randomized trial. Am J Clin Nutr. 2014; 100:1322–1328. PMID:
25332329.
9. Weber M, Grote V, Closa-Monasterolo R, Escribano J, Langhendries JP, Dain E, Giovannini M, Verduci E, Gruszfeld D, Socha P, et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr. 2014; 99:1041–1051. PMID:
24622805.
10. Ziegler EE, Fields DA, Chernausek SD, Steenhout P, Grathwohl D, Jeter JM, Nelson SE, Haschke F. Adequacy of infant formula with protein content of 1.6 g/100 kcal for infants between 3 and 12 months. J Pediatr Gastroenterol Nutr. 2015; 61:596–603. PMID:
26154030.
11. Monasta L, Batty GD, Cattaneo A, Lutje V, Ronfani L, Van Lenthe FJ, Brug J. Early-life determinants of overweight and obesity: a review of systematic reviews. Obes Rev. 2010; 11:695–708. PMID:
20331509.
12. Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M, Beyer J, Demmelmair H, Anton B, Gruszfeld D, et al. Can infant feeding choices modulate later obesity risk? Am J Clin Nutr. 2009; 89:1502S–1508S. PMID:
19321574.
13. Metges CC, Barth CA. Metabolic consequences of a high dietary-protein intake in adulthood: assessment of the available evidence. J Nutr. 2000; 130:886–889. PMID:
10736347.
14. Pedersen AN, Kondrup J, Børsheim E. Health effects of protein intake in healthy adults: a systematic literature review. Food Nutr Res. 2013; 57:23364.
15. Kim HH, Kim YJ, Lee SY, Jeong DW, Lee JG, Yi YH, Cho YH, Choi EJ, Kim HJ. Interactive effects of an isocaloric high-protein diet and resistance exercise on body composition, ghrelin, and metabolic and hormonal parameters in untrained young men: a randomized clinical trial. J Diabetes Investig. 2014; 5:242–247.
16. Gulati S, Misra A, Tiwari R, Sharma M, Pandey RM, Yadav CP. Effect of high-protein meal replacement on weight and cardiometabolic profile in overweight/obese Asian Indians in North India. Br J Nutr. 2017; 117:1531–1540. PMID:
28653586.
17. Rietman A, Schwarz J, Blokker BA, Siebelink E, Kok FJ, Afman LA, Tomé D, Mensink M. Increasing protein intake modulates lipid metabolism in healthy young men and women consuming a high-fat hypercaloric diet. J Nutr. 2014; 144:1174–1180. PMID:
24899158.
18. Campos-Nonato I, Hernandez L, Barquera S. Effect of a high-protein diet versus standard-protein diet on weight loss and biomarkers of metabolic syndrome: a randomized clinical trial. Obes Facts. 2017; 10:238–251. PMID:
28601864.
19. Preis SR, Stampfer MJ, Spiegelman D, Willett WC, Rimm EB. Dietary protein and risk of ischemic heart disease in middle-aged men. Am J Clin Nutr. 2010; 92:1265–1272. PMID:
20881068.
20. Prentice RL, Huang Y, Kuller LH, Tinker LF, Horn LV, Stefanick ML, Sarto G, Ockene J, Johnson KC. Biomarker-calibrated energy and protein consumption and cardiovascular disease risk among postmenopausal women. Epidemiology. 2011; 22:170–179. PMID:
21206366.
21. Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, Hu FB. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006; 355:1991–2002. PMID:
17093250.
22. Assmann KE, Joslowski G, Buyken AE, Cheng G, Remer T, Kroke A, Günther AL. Prospective association of protein intake during puberty with body composition in young adulthood. Obesity (Silver Spring). 2013; 21:E782–E789. PMID:
23788493.
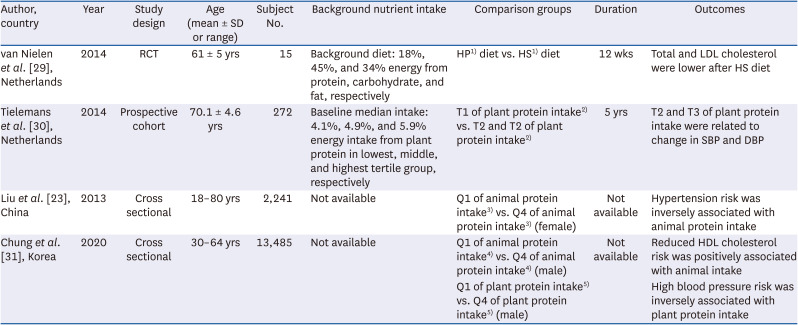
23. Liu R, Dang S, Yan H, Wang D, Zhao Y, Li Q, Liu X. Association between dietary protein intake and the risk of hypertension: a cross-sectional study from rural western China. Hypertens Res. 2013; 36:972–979. PMID:
23842622.
24. Oh C, No JK. Appropriate protein intake is one strategy in the management of metabolic syndrome in Korean elderly to mitigate changes in body composition. Nutr Res. 2018; 51:21–28. PMID:
29673541.
25. Friedman M. Nutritional value of proteins from different food sources. J Agric Food Chem. 1996; 44:6–29.
26. Altorf-van der Kuil W, Engberink MF, Vedder MM, Boer JM, Verschuren WM, Geleijnse JM. Sources of dietary protein in relation to blood pressure in a general Dutch population. PLoS One. 2012; 7:e30582. PMID:
22347387.
27. Wang YF, Yancy WS Jr, Yu D, Champagne C, Appel LJ, Lin PH. The relationship between dietary protein intake and blood pressure: results from the PREMIER study. J Hum Hypertens. 2008; 22:745–754. PMID:
18580887.
28. Umesawa M, Sato S, Imano H, Kitamura A, Shimamoto T, Yamagishi K, Tanigawa T, Iso H. Relations between protein intake and blood pressure in Japanese men and women: the Circulatory Risk in Communities Study (CIRCS). Am J Clin Nutr. 2009; 90:377–384. PMID:
19515740.
29. van Nielen M, Feskens EJ, Rietman A, Siebelink E, Mensink M. Partly replacing meat protein with soy protein alters insulin resistance and blood lipids in postmenopausal women with abdominal obesity. J Nutr. 2014; 144:1423–1429. PMID:
25008579.
30. Tielemans SM, Kromhout D, Altorf-van der Kuil W, Geleijnse JM. Associations of plant and animal protein intake with 5-year changes in blood pressure: the Zutphen Elderly Study. Nutr Metab Cardiovasc Dis. 2014; 24:1228–1233. PMID:
24998077.
31. Chung S, Chung MY, Choi HK, Park JH, Hwang JT, Joung H. Animal protein intake is positively associated with metabolic syndrome risk factors in middle-aged Korean men. Nutrients. 2020; 12:3415.
32. Roubenoff R. Sarcopenia: a major modifiable cause of frailty in the elderly. J Nutr Health Aging. 2000; 4:140–142. PMID:
10936900.
33. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56:M146–M156. PMID:
11253156.
34. Dennison EM, Sayer AA, Cooper C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat Rev Rheumatol. 2017; 13:340–347. PMID:
28469267.
35. Rolland Y, Abellan van Kan G, Bénétos A, Blain H, Bonnefoy M, Chassagne P, Jeandel C, Laroche M, Nourhashémi F, Orcel P, et al. Frailty, osteoporosis and hip fracture: causes, consequences and therapeutic perspectives. J Nutr Health Aging. 2008; 12:335–346. PMID:
18443717.
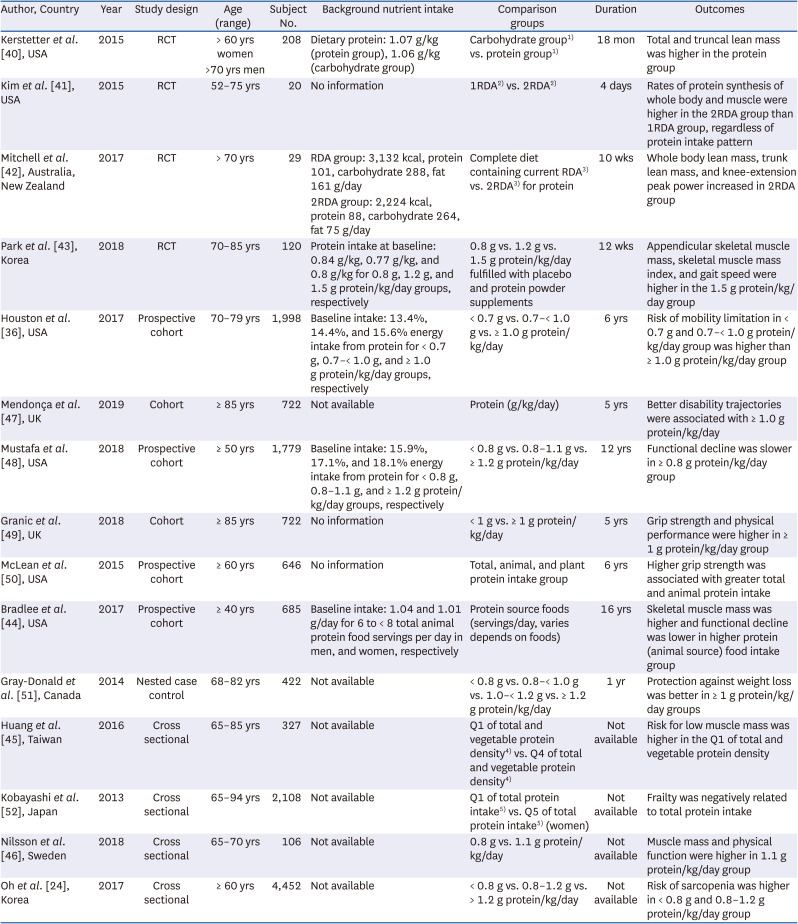
36. Houston DK, Tooze JA, Garcia K, Visser M, Rubin S, Harris TB, Newman AB, Kritchevsky SB. Health ABC Study. Protein intake and mobility limitation in community-dwelling older adults: the health ABC study. J Am Geriatr Soc. 2017; 65:1705–1711. PMID:
28306154.
37. Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005; 19:422–424. PMID:
15596483.
38. Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009; 587:211–217. PMID:
19001042.
39. Volpi E, Campbell WW, Dwyer JT, Johnson MA, Jensen GL, Morley JE, Wolfe RR. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci. 2013; 68:677–681. PMID:
23183903.
40. Kerstetter JE, Bihuniak JD, Brindisi J, Sullivan RR, Mangano KM, Larocque S, Kotler BM, Simpson CA, Cusano AM, Gaffney-Stomberg E, et al. The effect of a whey protein supplement on bone mass in older Caucasian adults. J Clin Endocrinol Metab. 2015; 100:2214–2222. PMID:
25844619.
41. Kim IY, Schutzler S, Schrader A, Spencer H, Kortebein P, Deutz NE, Wolfe RR, Ferrando AA. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am J Physiol Endocrinol Metab. 2015; 308:E21–E28. PMID:
25352437.
42. Mitchell CJ, Milan AM, Mitchell SM, Zeng N, Ramzan F, Sharma P, Knowles SO, Roy NC, Sjödin A, Wagner KH, et al. The effects of dietary protein intake on appendicular lean mass and muscle function in elderly men: a 10-wk randomized controlled trial. Am J Clin Nutr. 2017; 106:1375–1383. PMID:
29092886.
43. Park Y, Choi JE, Hwang HS. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2018; 108:1026–1033. PMID:
30475969.
44. Bradlee ML, Mustafa J, Singer MR, Moore LL. High-protein foods and physical activity protect against age-related muscle loss and functional decline. J Gerontol A Biol Sci Med Sci. 2017; 73:88–94. PMID:
28549098.
45. Huang RY, Yang KC, Chang HH, Lee LT, Lu CW, Huang KC. The association between total protein and vegetable protein intake and low muscle mass among the community-dwelling elderly population in Northern Taiwan. Nutrients. 2016; 8:8.
46. Nilsson A, Montiel Rojas D, Kadi F. Impact of meeting different guidelines for protein intake on muscle mass and physical function in physically active older women. Nutrients. 2018; 10:1156.
47. Mendonça N, Granic A, Hill TR, Siervo M, Mathers JC, Kingston A, Jagger C. Protein intake and disability trajectories in very old adults: the newcastle 85+ study. J Am Geriatr Soc. 2019; 67:50–56. PMID:
30382594.
48. Mustafa J, Ellison RC, Singer MR, Bradlee ML, Kalesan B, Holick MF, Moore LL. Dietary protein and preservation of physical functioning among middle-aged and older adults in the Framingham offspring study. Am J Epidemiol. 2018; 187:1411–1419. PMID:
29590270.
49. Granic A, Mendonça N, Sayer AA, Hill TR, Davies K, Adamson A, Siervo M, Mathers JC, Jagger C. Low protein intake, muscle strength and physical performance in the very old: the newcastle 85+ study. Clin Nutr. 2018; 37:2260–2270. PMID:
29191494.
50. McLean RR, Mangano KM, Hannan MT, Kiel DP, Sahni S. Dietary protein intake is protective against loss of grip strength among older adults in the Framingham offspring cohort. J Gerontol A Biol Sci Med Sci. 2016; 71:356–361. PMID:
26525088.
51. Gray-Donald K, St-Arnaud-McKenzie D, Gaudreau P, Morais JA, Shatenstein B, Payette H. Protein intake protects against weight loss in healthy community-dwelling older adults. J Nutr. 2014; 144:321–326. PMID:
24357473.
52. Kobayashi S, Asakura K, Suga H, Sasaki S. Three-generation Study of Women on Diets and Health Study Group. High protein intake is associated with low prevalence of frailty among old Japanese women: a multicenter cross-sectional study. Nutr J. 2013; 12:164. PMID:
24350714.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download