1. Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog Horm Res. 2002; 57:257–275. PMID:
12017547.
2. Barnabei VM, Grady D, Stovall DW, Cauley JA, Lin F, Stuenkel CA, Stefanick ML, Pickar JH. Menopausal symptoms in older women and the effects of treatment with hormone therapy. Obstet Gynecol. 2002; 100:1209–1218. PMID:
12468165.
3. Hulley S, Furberg C, Barrett-Connor E, Cauley J, Grady D, Haskell W, Knopp R, Lowery M, Satterfield S, Schrott H, et al. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002; 288:58–66. PMID:
12090863.
4. Nachtigall LE, Nachtigall RH, Nachtigall RD, Beckman EM. Estrogen replacement therapy II: a prospective study in the relationship to carcinoma and cardiovascular and metabolic problems. Obstet Gynecol. 1979; 54:74–79. PMID:
221871.
5. Cos P, De Bruyne T, Apers S, Vanden Berghe D, Pieters L, Vlietinck AJ. Phytoestrogens: recent developments. Planta Med. 2003; 69:589–599. PMID:
12898412.
6. Moutsatsou P. The spectrum of phytoestrogens in nature: our knowledge is expanding. Hormones (Athens). 2007; 6:173–193. PMID:
17724002.
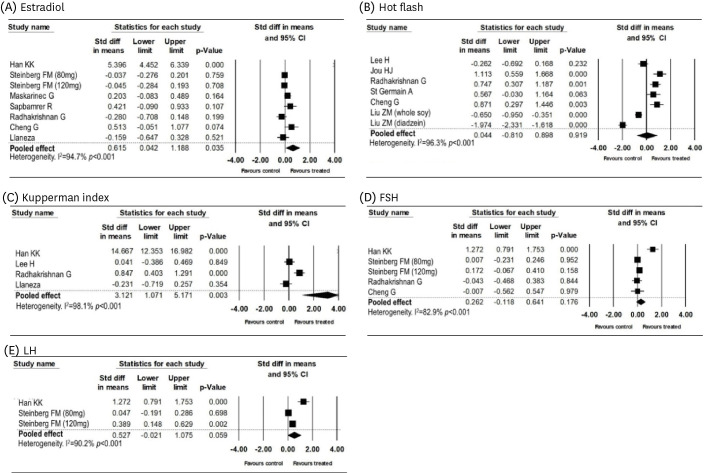
7. Pilšáková L, Riečanský I, Jagla F. The physiological actions of isoflavone phytoestrogens. Physiol Res. 2010; 59:651–664. PMID:
20406033.
8. Ewies AA. Phytoestrogens in the management of the menopause: up-to-date. Obstet Gynecol Surv. 2002; 57:306–313. PMID:
11997677.
9. Franco OH, Chowdhury R, Troup J, Voortman T, Kunutsor S, Kavousi M, Oliver-Williams C, Muka T. Use of plant-based therapies and menopausal symptoms: a systematic review and meta-analysis. JAMA. 2016; 315:2554–2563. PMID:
27327802.
10. Chen MN, Lin CC, Liu CF. Efficacy of phytoestrogens for menopausal symptoms: a meta-analysis and systematic review. Climacteric. 2015; 18:260–269. PMID:
25263312.
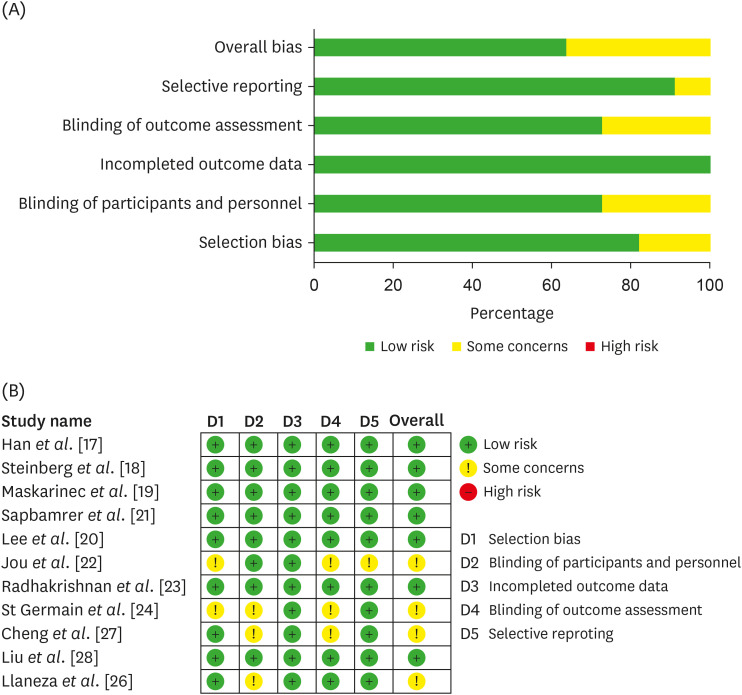
11. Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. London: The Cochrane Collaboration;2019. p. 205–228.
12. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–1558. PMID:
12111919.
13. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954; 10:101–129.
14. Page MJ, Higgins JP, Sterne JA. Assessing risk of bias due to missing results in a synthesis. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. London: The Cochrane Collaboration;2019. p. 349–374.
15. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634. PMID:
9310563.
16. Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Ch 5.4. Outlier & influential cases. Harrer M, Cuijpers P, Furukawa TA, Ebert DD, editors. Doing Meta-Analysis with R: a Hands-on Guide. London: Chapman and Hall/CRC;2021. p. 153.
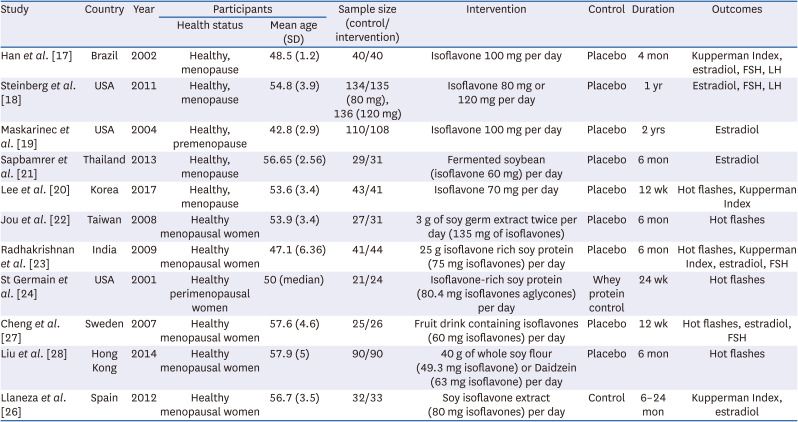
17. Han KK, Soares JM Jr, Haidar MA, de Lima GR, Baracat EC. Benefits of soy isoflavone therapeutic regimen on menopausal symptoms. Obstet Gynecol. 2002; 99:389–394. PMID:
11864664.
18. Steinberg FM, Murray MJ, Lewis RD, Cramer MA, Amato P, Young RL, Barnes S, Konzelmann KL, Fischer JG, Ellis KJ, et al. Clinical outcomes of a 2-y soy isoflavone supplementation in menopausal women. Am J Clin Nutr. 2011; 93:356–367. PMID:
21177797.
19. Maskarinec G, Franke AA, Williams AE, Hebshi S, Oshiro C, Murphy S, Stanczyk FZ. Effects of a 2-year randomized soy intervention on sex hormone levels in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2004; 13:1736–1744. PMID:
15533901.
20. Lee H, Choue R, Lim H. Effect of soy isoflavones supplement on climacteric symptoms, bone biomarkers, and quality of life in Korean postmenopausal women: a randomized clinical trial. Nutr Res Pract. 2017; 11:223–231. PMID:
28584579.
21. Sapbamrer R, Visavarungroj N, Suttajit M. Effects of dietary traditional fermented soybean on reproductive hormones, lipids, and glucose among postmenopausal women in northern Thailand. Asia Pac J Clin Nutr. 2013; 22:222–228. PMID:
23635365.
22. Jou HJ, Wu SC, Chang FW, Ling PY, Chu KS, Wu WH. Effect of intestinal production of equol on menopausal symptoms in women treated with soy isoflavones. Int J Gynaecol Obstet. 2008; 102:44–49. PMID:
18395723.
23. Radhakrishnan G, Rashmi NA, Vaid NB. Evaluation of isoflavone rich soy protein supplementation for postmenopausal therapy. Pak J Nutr. 2009; 8:1009–1017.
24. St Germain A, Peterson CT, Robinson JG, Alekel DL. Isoflavone-rich or isoflavone-poor soy protein does not reduce menopausal symptoms during 24 weeks of treatment. Menopause. 2001; 8:17–26. PMID:
11201510.
25. Liu ZM, Chen YM, Ho SC, Ho YP, Woo J. Effects of soy protein and isoflavones on glycemic control and insulin sensitivity: a 6-mo double-blind, randomized, placebo-controlled trial in postmenopausal Chinese women with prediabetes or untreated early diabetes. Am J Clin Nutr. 2010; 91:1394–1401. PMID:
20335543.
26. Llaneza P, González C, Fernández-Iñarrea J, Alonso A, Díaz F, Pérez-López FR. Soy isoflavones improve insulin sensitivity without changing serum leptin among postmenopausal women. Climacteric. 2012; 15:611–620. PMID:
22191384.
27. Cheng G, Wilczek B, Warner M, Gustafsson JA, Landgren BM. Isoflavone treatment for acute menopausal symptoms. Menopause. 2007; 14:468–473. PMID:
17290160.
28. Liu ZM, Ho SC, Woo J, Chen YM, Wong C. Randomized controlled trial of whole soy and isoflavone daidzein on menopausal symptoms in equol-producing Chinese postmenopausal women. Menopause. 2014; 21:653–660. PMID:
24149925.
29. Lambert MN, Thorup AC, Hansen ES, Jeppesen PB. Combined red clover isoflavones and probiotics potently reduce menopausal vasomotor symptoms. PLoS One. 2017; 12:e0176590. PMID:
28591133.
30. Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, Phipps WR, Cassidy A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009; 15:423–440. PMID:
19299447.
31. Fritz H, Seely D, Flower G, Skidmore B, Fernandes R, Vadeboncoeur S, Kennedy D, Cooley K, Wong R, Sagar S, et al. Soy, red clover, and isoflavones and breast cancer: a systematic review. PLoS One. 2013; 8:e81968. PMID:
24312387.
32. Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann Intern Med. 2002; 137:805–813. PMID:
12435217.
33. Haines CJ, Xing SM, Park KH, Holinka CF, Ausmanas MK. Prevalence of menopausal symptoms in different ethnic groups of Asian women and responsiveness to therapy with three doses of conjugated estrogens/medroxyprogesterone acetate: the Pan-Asia Menopause (PAM) study. Maturitas. 2005; 52:264–276. PMID:
15921865.
34. Shea JL. Parsing the ageing Asian woman: symptom results from the China study of midlife women. Maturitas. 2006; 55:36–50. PMID:
16472950.
35. Clarkson TB, Utian WH, Barnes S, Gold EB, Basaria SS, Aso T, Kronenberg F, Frankenfeld CL, Cline JM, Landgren BM, et al. The role of soy isoflavones in menopausal health: report of The North American Menopause Society/Wulf H. Utian Translational Science Symposium in Chicago, IL (October 2010). Menopause. 2011; 18:732–753. PMID:
21685820.
36. Li N, Wu X, Zhuang W, Xia L, Chen Y, Zhao R, Yi M, Wan Q, Du L, Zhou Y. Soy and isoflavone consumption and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomized trials in humans. Mol Nutr Food Res. 2020; 64:e1900751. PMID:
31584249.
37. Thomas AJ, Ismail R, Taylor-Swanson L, Cray L, Schnall JG, Mitchell ES, Woods NF. Effects of isoflavones and amino acid therapies for hot flashes and co-occurring symptoms during the menopausal transition and early postmenopause: a systematic review. Maturitas. 2014; 78:263–276. PMID:
24951101.
38. Chen LR, Ko NY, Chen KH. Isoflavone supplements for menopausal women: a systematic review. Nutrients. 2019; 11:E2649. PMID:
31689947.
39. Daily JW, Ko BS, Ryuk J, Liu M, Zhang W, Park S. Equol decreases hot flashes in postmenopausal women: a systematic review and meta-analysis of randomized clinical trials. J Med Food. 2019; 22:127–139. PMID:
30592686.
40. Ghazanfarpour M, Latifnejad Roudsari R, Treglia G, Sadeghi R. Topical administration of isoflavones for treatment of vaginal symptoms in postmenopausal women: a systematic review of randomised controlled trials. J Obstet Gynaecol. 2015; 35:783–787. PMID:
25710207.
41. Brink E, Coxam V, Robins S, Wahala K, Cassidy A, Branca F. PHYTOS Investigators. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: a randomized, double-blind, placebo controlled study. Am J Clin Nutr. 2008; 87:761–770. PMID:
18326616.
42. Ryan-Borchers TA, Park JS, Chew BP, McGuire MK, Fournier LR, Beerman KA. Soy isoflavones modulate immune function in healthy postmenopausal women. Am J Clin Nutr. 2006; 83:1118–1125. PMID:
16685055.
43. Tai TY, Tsai KS, Tu ST, Wu JS, Chang CI, Chen CL, Shaw NS, Peng HY, Wang SY, Wu CH. The effect of soy isoflavone on bone mineral density in postmenopausal Taiwanese women with bone loss: a 2-year randomized double-blind placebo-controlled study. Osteoporos Int. 2012; 23:1571–1580. PMID:
21901480.
44. Charles C, Yuskavage J, Carlson O, John M, Tagalicud AS, Maggio M, Muller DC, Egan J, Basaria S. Effects of high-dose isoflavones on metabolic and inflammatory markers in healthy postmenopausal women. Menopause. 2009; 16:395–400. PMID:
18981951.
45. Chilibeck PD, Vatanparast H, Pierson R, Case A, Olatunbosun O, Whiting SJ, Beck TJ, Pahwa P, Biem HJ. Effect of exercise training combined with isoflavone supplementation on bone and lipids in postmenopausal women: a randomized clinical trial. J Bone Miner Res. 2013; 28:780–793. PMID:
23165609.
46. Basaria S, Wisniewski A, Dupree K, Bruno T, Song MY, Yao F, Ojumu A, John M, Dobs AS. Effect of high-dose isoflavones on cognition, quality of life, androgens, and lipoprotein in post-menopausal women. J Endocrinol Invest. 2009; 32:150–155. PMID:
19411814.
47. Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR, Krischer J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: a randomized, double-blind trial. Arch Intern Med. 2011; 171:1363–1369. PMID:
21824950.
48. Valentine JC, Pigott TD, Rothstein HR. How many studies do you need? A primer on statistical power for meta-analysis (vol 35, pg 215, 2010). J Educ Behav Stat. 2010; 35:375.
49. Weare K, Nind M. Mental health promotion and problem prevention in schools: what does the evidence say? Health Promot Int. 2011; 26(Suppl 1):i29–i69. PMID:
22079935.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download