1. Ellingson DJ, Shippar JJ, Gilmore JM. Determination of free and total choline and carnitine in infant formula and adult/pediatric nutritional formula by liquid chromatography/tandem mass spectrometry (LC/MS/MS): single-laboratory validation, first action 2015.10. J AOAC Int. 2016; 99:204–209. PMID:
26822979.
2. Bremer J, Greenberg D. Methyl transfering enzyme system of microsomes in the biosynthesis of lecithin (phosphatidylcholine). Biochim Biophys Acta. 1961; 46:205–216.
3. Zeisel SH, Klatt KC, Caudill MA. Choline. Adv Nutr. 2018; 9:58–60. PMID:
29438456.
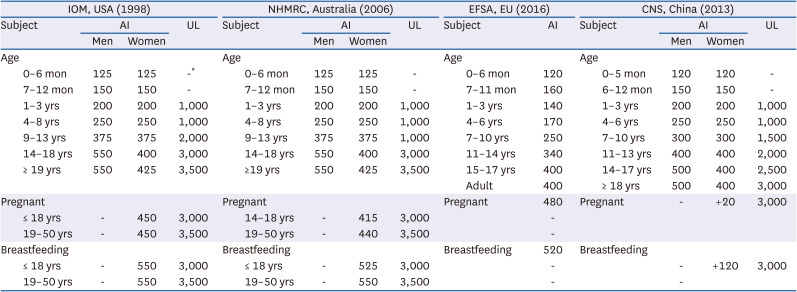
4. Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, D.C.: National Academies Press;1998. p. 390–422.
5. Australian Government Department of Health and Ageing, New Zealand Ministry of Health. National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand: Including Recommended Dietary Intakes. Canberra: National Health and Medical Research Council;2006.
6. Chinese Nutrition Society. Chinese Dietary Reference Intakes 2013. Beijing: China Science Publishing and Media Ltd.;2013.
7. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Dietary reference values for choline. EFSA J. 2016; 14:4484.
8. Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. 1994; 14:269–296. PMID:
7946521.
9. Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006; 26:229–250. PMID:
16848706.
10. Stead LM, Brosnan JT, Brosnan ME, Vance DE, Jacobs RL. Is it time to reevaluate methyl balance in humans? Am J Clin Nutr. 2006; 83:5–10. PMID:
16400042.
11. Buchman AL, Dubin MD, Moukarzel AA, Jenden DJ, Roch M, Rice KM, Gornbein J, Ament ME. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology. 1995; 22:1399–1403. PMID:
7590654.
12. Fischer LM, daCosta KA, Kwock L, Stewart PW, Lu TS, Stabler SP, Allen RH, Zeisel SH. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr. 2007; 85:1275–1285. PMID:
17490963.
13. Dalmeijer GW, Olthof MR, Verhoef P, Bots ML, van der Schouw YT. Prospective study on dietary intakes of folate, betaine, and choline and cardiovascular disease risk in women. Eur J Clin Nutr. 2008; 62:386–394. PMID:
17375117.
14. Konstantinova SV, Tell GS, Vollset SE, Nygård O, Bleie Ø, Ueland PM. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr. 2008; 138:914–920. PMID:
18424601.
15. Bartus RT, Dean RL 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982; 217:408–414. PMID:
7046051.
16. Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004; 160:102–109. PMID:
15234930.
17. Xu X, Gammon MD, Zeisel SH, Lee YL, Wetmur JG, Teitelbaum SL, Bradshaw PT, Neugut AI, Santella RM, Chen J. Choline metabolism and risk of breast cancer in a population-based study. FASEB J. 2008; 22:2045–2052. PMID:
18230680.
18. Xu X, Gammon MD, Zeisel SH, Bradshaw PT, Wetmur JG, Teitelbaum SL, Neugut AI, Santella RM, Chen J. High intakes of choline and betaine reduce breast cancer mortality in a population-based study. FASEB J. 2009; 23:4022–4028. PMID:
19635752.
19. Lee JE, Giovannucci E, Fuchs CS, Willett WC, Zeisel SH, Cho E. Choline and betaine intake and the risk of colorectal cancer in men. Cancer Epidemiol Biomarkers Prev. 2010; 19:884–887. PMID:
20160273.
20. Zeisel SH, Da Costa KA, Franklin PD, Alexander EA, Lamont JT, Sheard NF, Beiser A. Choline, an essential nutrient for humans. FASEB J. 1991; 5:2093–2098. PMID:
2010061.
21. Holmes-McNary MQ, Cheng WL, Mar MH, Fussell S, Zeisel SH. Choline and choline esters in human and rat milk and in infant formulas. Am J Clin Nutr. 1996; 64:572–576. PMID:
8839502.
22. Zeisel SH, Char D, Sheard NF. Choline, phosphatidylcholine and sphingomyelin in human and bovine milk and infant formulas. J Nutr. 1986; 116:50–58. PMID:
3944656.
23. National Center for Health Statistics (US). Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994; (32):1–407.
24. WHO Multicentre Growth Reference Study Group. Breastfeeding in the WHO Multicentre Growth Reference Study. Acta Paediatr Suppl. 2006; 450:16–26. PMID:
16817675.
25. West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997; 276:122–126. PMID:
9082983.
26. Kleiber M. Body size and metabolic rate. Physiol Rev. 1947; 27:511–541. PMID:
20267758.
27. Vennemann FB, Ioannidou S, Valsta LM, Dumas C, Ocké MC, Mensink GB, Lindtner O, Virtanen SM, Tlustos C, D’Addezio L, et al. Dietary intake and food sources of choline in European populations. Br J Nutr. 2015; 114:2046–2055. PMID:
26423357.
28. Widdowson EM, McCance RA. The effect of finite periods of undernutrition at different ages on the composition and subsequent development of the rat. Proc R Soc Lond B Biol Sci. 1963; 158:329–342. PMID:
14070049.
29. Welsch F. Studies on accumulation and metabolic fate of (N-Me3h)choline in human term placenta fragments. Biochem Pharmacol. 1976; 25:1021–1030. PMID:
1267847.
30. Boyd WD, Graham-White J, Blackwood G, Glen I, McQueen J. Clinical effects of choline in Alzheimer senile dementia. Lancet. 1977; 2:711.
31. Gelenberg AJ, Doller-Wojcik JC, Growdon JH. Choline and lecithin in the treatment of tardive dyskinesia: preliminary results from a pilot study. Am J Psychiatry. 1979; 136:772–776. PMID:
375755.
32. Growdon JH, Cohen EL, Wurtman RJ. Huntington’s disease: clinical and chemical effects of choline administration. Ann Neurol. 1977; a. 1:418–422. PMID:
152596.
33. Growdon JH, Hirsch MJ, Wurtman RJ, Wiener W. Oral choline administration to patients with tardive dyskinesia. N Engl J Med. 1977; b. 297:524–527. PMID:
887103.
34. Lawrence CM, Millac P, Stout GS, Ward JW. The use of choline chloride in ataxic disorders. J Neurol Neurosurg Psychiatry. 1980; 43:452–454. PMID:
6999128.
35. Jeong HO, Kim CI, Lee HS, Chung YJ. Estimation of dietary choline intake of Korean by gender, age and region. Korean J Nutr. 2005; 38:320–326.
36. Chung YJ, Cho HJ, Na JS. Dietary choline intake of Korean young adults. Korean J Nutr. 2004; 37:61–67.
37. Korea Food and Drug Administration. Korea Health Industry Development Institute. Dietary Intake and Risk Assessment of Contaminants in Korean Foods. Seoul: Korea Health Industry Development Institute;2006.
38. Na JS, Cho HJ, Lim JH, Yun HI, Sok DE, Lee JW, Byun MW, Chung YJ. Plasma choline concentration of some Korean young adults and correlation with dietary choline intake. Korean J Nutr. 2006; 39:115–120.
39. Wiedeman AM, Barr SI, Green TJ, Xu Z, Innis SM, Kitts DD. Dietary choline intake: current state of knowledge across the life cycle. Nutrients. 2018; 10:1513.
40. Yu D, Shu XO, Xiang YB, Li H, Yang G, Gao YT, Zheng W, Zhang X. Higher dietary choline intake is associated with lower risk of nonalcoholic fatty liver in normal-weight Chinese women. J Nutr. 2014; 144:2034–2040. PMID:
25320186.
41. López-Carrillo L, Gamboa-Loira B, Becerra W, Hernández-Alcaraz C, Hernández-Ramírez RU, Gandolfi AJ, Franco-Marina F, Cebrián ME. Dietary micronutrient intake and its relationship with arsenic metabolism in Mexican women. Environ Res. 2016; 151:445–450. PMID:
27565879.
42. Cheng CP, Chen CH, Kuo CS, Kuo HT, Huang KT, Shen YL, Chang CH, Huang RF. Dietary choline and folate relationships with serum hepatic inflammatory injury markers in Taiwanese adults. Asia Pac J Clin Nutr. 2017; 26:642–649. PMID:
28582814.
43. Nagata C, Wada K, Tamura T, Konishi K, Kawachi T, Tsuji M, Nakamura K. Choline and betaine intakes are not associated with cardiovascular disease mortality risk in Japanese men and women. J Nutr. 2015; 145:1787–1792. PMID:
26063062.
44. Yonemori KM, Lim U, Koga KR, Wilkens LR, Au D, Boushey CJ, Le Marchand L, Kolonel LN, Murphy SP. Dietary choline and betaine intakes vary in an adult multiethnic population. J Nutr. 2013; 143:894–899. PMID:
23616508.
45. Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, Stram DO, Monroe KR, Earle ME, Nagamine FS. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000; 151:346–357. PMID:
10695593.