INTRODUCTION
MATERIALS AND METHODS
Materials
Animals and experimental design
 | Fig. 1Experimental design to examine the effects of GPE, GL, CrM supplementation on exercise adaptation. The animals were assigned randomly to the indicated 6 groups (n = 10 per group in each test). Physical exercise capacity and related assessments were conducted during the test for 6 weeks.GPE, G. pentaphyllum extract; GL, gypenoside L; BW, body weight; CrM, creatine monohydrate; SC, sedentary with vehicle; SGPE, sedentary with 300 mg/kg BW/day of GPE; ExC, exercise with vehicle; ExGPE, exercise with 300 mg/kg BW/day of GPE; ExGL, exercise with 7 mg/kg BW/day of GL; ExCrM, exercise with 75 mg/kg BW/day of CrM.
|
Exercise endurance test
Body composition analysis and sample collection
Blood chemistry assay
Tissue glycogen content assay
Protein expression analysis
Real-time reverse-transcription polymerase chain reaction (RT-PCR)
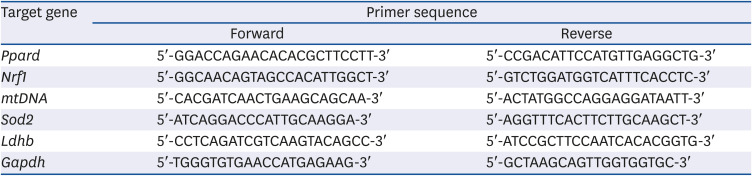
Table 1
Quantitative real-time polymerase chain reaction primers
Statistical analysis
RESULTS
Effects of GPE administration on the body and muscle weight in treadmill-trained mice
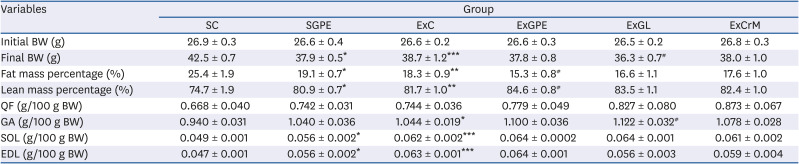
Table 2
Effect of GPE and GL treatment on BW, fat mass percentage, lean mass percentage, and QF, GA, SOL, and EDL weights in treadmill-trained ICR mice
Effect of GPE administration on the endurance exercise performance in the treadmill-trained mice
 | Fig. 2Effects of GPE and GL treatment on treadmill exercise performance. (A) Endurance time to exhaustion and (B) exercise capacity. Values are means ± SEM for n = 10 per group. GPE, G. pentaphyllum extract; GL, gypenoside L; BW, body weight; CrM, creatine monohydrate; SC, sedentary with vehicle; SGPE, sedentary with 300 mg/kg BW/day of GPE; ExC, exercise with vehicle; ExGPE, exercise with 300 mg/kg BW/day of GPE; ExGL, exercise with 7 mg/kg BW/day of GL; ExCrM, exercise with 75 mg/kg BW/day of CrM.
***P < 0.001 (SC group vs. SGPE or ExC group); #P < 0.05, ##P < 0.01, ###P < 0.001 (ExC group vs. ExGPE, ExGL or ExCrM group).
|
Effect of GPE administration on fatigue-associated biochemistry
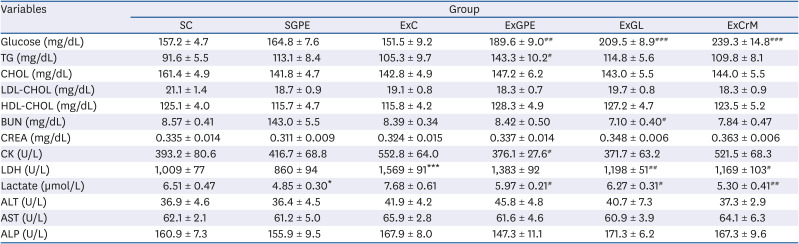
Table 3
Effects of GPE and GL treatment on clinical biochemistry tests in ICR mice
Effect of GPE administration on the liver and muscle glycogen level
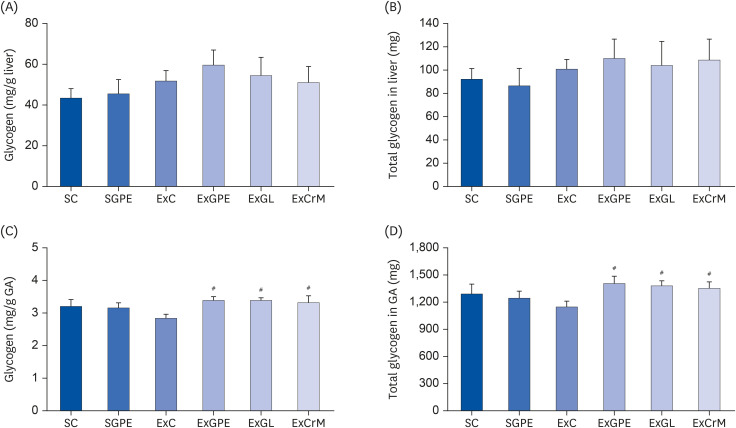 | Fig. 3Effects of GPE and GL treatment on glycogen contents in the liver and muscle of ICR mice. (A) Glycogen content (mg/g liver). (B) Total glycogen in liver (mg). (C) Glycogen content (mg/g GA). (D) Total glycogen in GA (mg). The values are the means ± SEM for n = 10 per group.GPE, G. pentaphyllum extract; GL, gypenoside L; BW, body weight; CrM, creatine monohydrate; SC, sedentary with vehicle; SGPE, sedentary with 300 mg/kg BW/day of GPE; ExC, exercise with vehicle; ExGPE, exercise with 300 mg/kg BW/day of GPE; ExGL, exercise with 7 mg/kg BW/day of GL; ExCrM, exercise with 75 mg/kg BW/day of CrM; GA, gastrocnemius muscle.
#P < 0.05 (ExC group vs. ExGPE, ExGL or ExCrM group).
|
Effect of GPE administration on mitochondrial biogenesis
 | Fig. 4Effects of the GPE and GL treatment on the protein and mRNA expression of mitochondrial biogenesis genes in the soleus muscle of ICR mice. (A) Protein levels of phosphorylated PGC-1α and PGC-1α were analyzed using a western blot assay. β-actin served as an internal control. The ratio of p-PGC-1α/PGC-1α was determined (n = 4 per group). The mRNA levels of Nrf1 (B), and mtDNA content (C) were analyzed by qRT-PCR. The target mRNA expression was normalized to Gapdh. The values are means ± SEM for n = 8–10 per group.GPE, G. pentaphyllum extract; GL, gypenoside L; BW, body weight; CrM, creatine monohydrate; SC, sedentary with vehicle; SGPE, sedentary with 300 mg/kg BW of GPE; ExC, exercise with vehicle; ExGPE, exercise with 300 mg/kg BW/day of GPE; ExGL, exercise with 7 mg/kg BW/day of GL; ExCrM, exercise with 75 mg/kg BW/day of CrM; p-PGC-1α, phosphorylated peroxisome proliferator-activated receptor γ coactivator 1-alpha; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1-alpha; Nrf1, nuclear respiratory factor 1; mtDNA, mitochondrial DNA; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; Gapdh, glyceraldehyde 3-phosphate dehydrogenase.
*P < 0.05, **P < 0.01, ***P < 0.001 (SC group vs. SGPE or ExC group); #P < 0.05, ##P < 0.01, ###P < 0.001 (ExC group vs. ExGPE, ExGL, or ExCrM group).
|
Effect of GPE administration on PGC-1α targeted genes expression
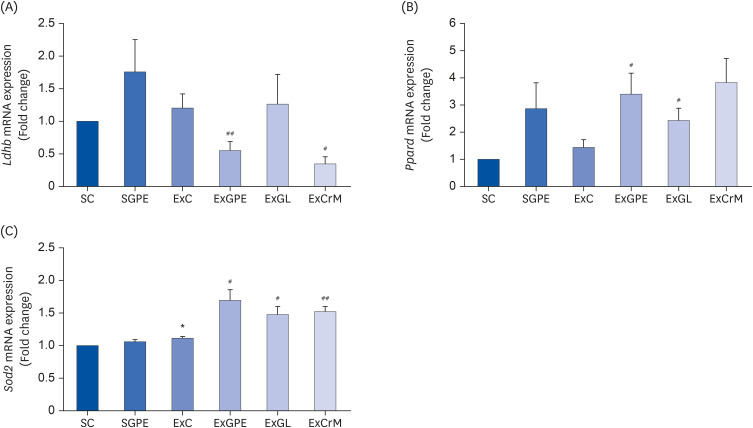 | Fig. 5Effects of the GPE and GL treatment on the mRNA expression of PGC-1α targeted genes in the soleus muscle of ICR mice. The mRNA levels of Ldhb (A), Ppard (B), and Sod2 (C) were analyzed by qRT-PCR. The target mRNA expression was normalized to Gapdh. Values are means ± SEM for n = 4–6 per group.GPE, G. pentaphyllum extract; GL, gypenoside L; BW, body weight; CrM, creatine monohydrate; SC, sedentary with vehicle; SGPE, sedentary with 300 mg/kg BW/day of GPE; ExC, exercise with vehicle; ExGPE, exercise with 300 mg/kg BW/day of GPE; ExGL, exercise with 7 mg/kg BW/day of GL; ExCrM, exercise with 75 mg/kg BW/day of CrM; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1-alpha; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; Ldhb, lactate dehydrogenase B; Ppard, peroxisome proliferator-activated receptor-δ; Sod2, superoxide dismutase 2; Gapdh, glyceraldehyde 3-phosphate dehydrogenase.
*P < 0.05 (SC group vs. SGPE or ExC group); #P < 0.05, ##P < 0.01 (ExC group vs. ExGPE, ExGL, or ExCrM group).
|
Effect of GPE administration on PGC-1α activation through AMPK and p38 phosphorylation
 | Fig. 6Effects of the GPE and GL treatment on PGC-1α activation through AMPK/p38 phosphorylation in soleus muscle of ICR mice. The protein levels of phosphorylated AMPK and AMPK (A) and phosphorylated p38, and p38 (B) were analyzed by western blot assay. β-actin served as an internal control. The ratio of p-AMPK/AMPK and p-p38/p38 were determined. The values are the means ± SEM for n = 4 per group.GPE, G. pentaphyllum extract; GL, gypenoside L; BW, body weight; CrM, creatine monohydrate; SC, sedentary with vehicle; SGPE, sedentary with 300 mg/kg BW/day of GPE; ExC, exercise with vehicle; ExGPE, exercise with 300 mg/kg BW/day of GPE; ExGL, exercise with 7 mg/kg BW/day of GL; ExCrM, exercise with 75 mg/kg BW/day of CrM; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1-alpha; p-AMPK, phosphorylated adenosine monophosphate-activated protein kinase; AMPK, adenosine monophosphate-activated protein kinase; p-p38, phosphorylated p38 MAP kinase; p38, p38 MAP kinase.
*P < 0.05 (SC group vs. SGPE or ExC group); #P < 0.05, ##P < 0.01, ###P < 0.001 (ExC group vs. ExGPE, ExGL, or ExCrM group).
|
DISCUSSION
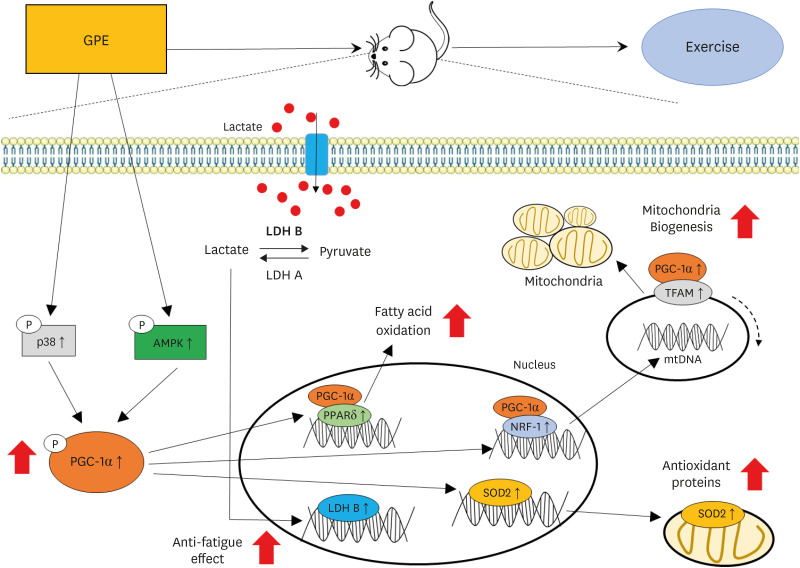 | Fig. 7Summary of the effects of the G. pentaphyllum extract on the exercise endurance and energy metabolism.GPE, G. pentaphyllum extract; p38, p38 MAP kinase; AMPK, adenosine monophosphate-activated protein kinase; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1-alpha; LDH B, lactate dehydrogenase B; LDH A, lactate dehydrogenase A; PPARδ, peroxisome proliferator-activated receptor-δ; NRF-1, nuclear respiratory factor 1; SOD2, superoxide dismutase 2; TFAM, transcription factor A mitochondrial; mtDNA, mitochondrial DNA.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download