INTRODUCTION
MATERIALS AND METHODS
Preparation of EEZS
Cell culture
Cell viability assay
Observation of apoptotic morphological changes
Assessment of apoptosis by flow cytometer
Protein extraction and Western blot analysis
Assay of caspase activity
Measurement of mitochondrial membrane potential (MMP)
Determination of ROS generation
Statistical analysis
RESULTS
Effect of EEZS on cell viability of T24 cells
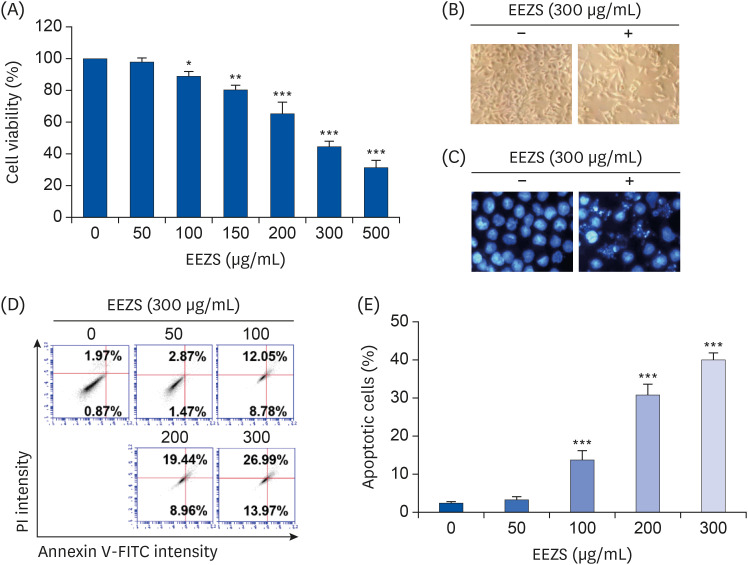 | Fig. 1Inhibition of cell survival and induction of apoptosis by EEZS in T24 cells. Cells were cultured for 48 h in medium containing the indicated concentrations of EEZS. (A) Cell viability was measured by MTT assay. (B) Morphological changes were observed with a phase-contrast microscope. (C) Nuclear morphological changes were observed under a fluorescence microscope using DAPI staining. (D) Flow cytometry analysis using annexin V/PI double staining was applied to measure the degree of apoptosis in T24 cells after EEZS treatment. (E) Statistical analysis of apoptotic cell population. The percentage of cells undergoing apoptosis is expressed as the percentage of the number of annexin V-positive cells using a flow cytometer. Data are expressed as the mean ± SD.EEZS, ethanol extracts of Z. schinifolium leaves; FITC, fluorescein isothiocyanate; PI, propidium iodine; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide; DAPI, 4′,6′-diamidino-2-phenylindole.
*P < 0.05, **P < 0.01 and ***P < 0.001 vs. untreated cells.
|
EEZs induces apoptosis in T24 cells
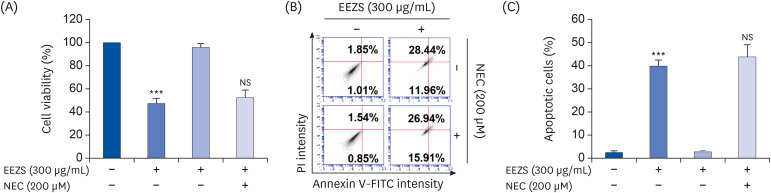 | Fig. 2Effect of necrostain-1, an inhibitor of necrosis, on decreased cell viability and increased apoptosis by EEZS in T24 cells. Cells were pre-treated with 200 μM NEC for 1 h, after which they were exposed to 300 μg/mL EEZS for 48 h. MTT assay (A) and flow cytometry analysis (B and C) were subsequently conducted. Data are expressed as the mean ± SD (n = 3).EEZS, ethanol extracts of Z. schinifolium leaves; NEC, necrostain-1; FITC, fluorescein isothiocyanate; PI, propidium iodine; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide.
***P < 0.001 vs. untreated cells; NS, not significant vs. 300 μg/mL EEZS-treated cells.
|
Effect of EEZS on the expression of DR-mediated proteins and activity of caspases in T24 cells
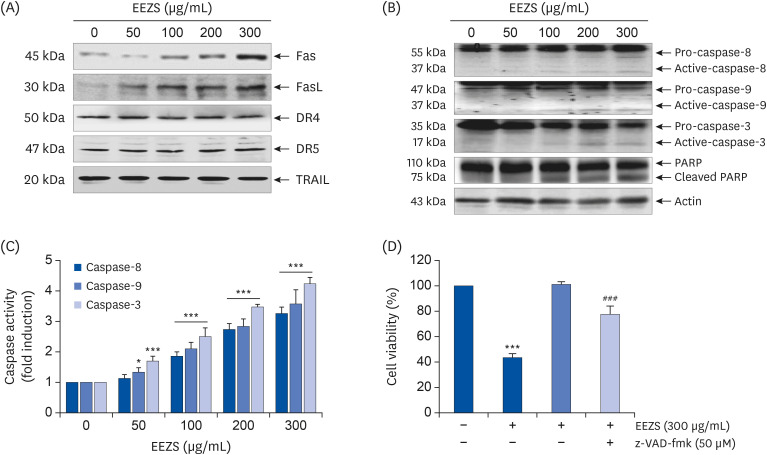 | Fig. 3Induction of Fas and FasL expression, and activation of caspases by EEZS in T24 cells. (A-C) Cells were cultured in media containing various concentrations of EEZS for 48 h. (A and B) Western blot analysis of indicated proteins. Actin was used as a control for protein loading. (C) The caspase activities in each sample were measured using caspase assay kits. (D) Cells were treated with 300 μg/mL EEZS for 48 h, with or without 50 μM z-VAD-fmk for 1 h. Cell viability was measured by the MTT assay. (C and D) Data are expressed as the mean ± SD.EEZS, ethanol extracts of Z. schinifolium leaves; FasL, Fas ligand; DR, death receptor; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; PARP, poly (ADP-ribose) polymerase; z-VAD-fmk, carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide.
*P < 0.05 and ***P < 0.001 vs. untreated cells. ###P < 0.001 vs. 300 μg/mL EEZS-treated cells.
|
EEZS regulates the levels of Bcl-2 family protein and increase mitochondrial dysfunction in T24 cells
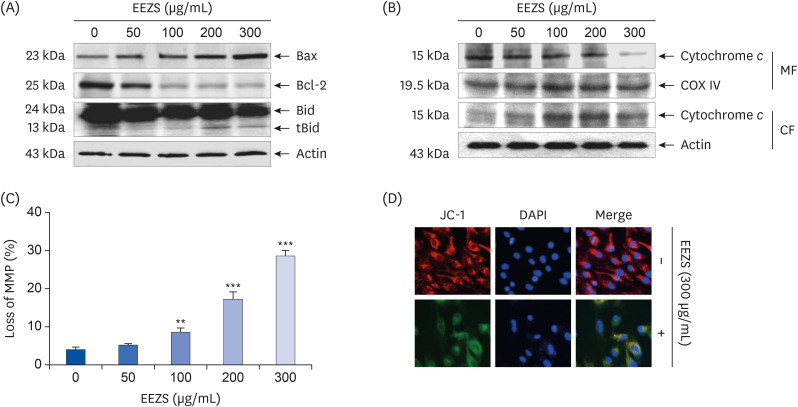 | Fig. 4Regulation of expression of Bcl-2 family proteins, cytosolic release of cytochrome c, and loss of MMP after EEZS exposure in T24 cells. Cells were cultured for 48 h in medium containing the indicated concentrations of EEZS. (A) Western blot analysis of Bcl-2 family proteins. Actin was used as a control for protein loading. (B) Mitochondrial and cytosolic fractions were extracted from cells, and cytochrome c expression was analyzed by Western blot analysis. The expression of COX VI and actin in each protein extract was used as a control for protein loading. (C) JC-1-stained cells were analyzed by flow cytometry for the extent of loss of MMP. Data are expressed as the mean ± SD. (D) Representative fluorescent images of T24 cells stained with JC-1 after 300 μg/mL EEZS treatment. Red color indicates healthy cells with high MMP, while green color indicates low MMP. DAPI was used to counterstain the nuclei (blue).EEZS, ethanol extracts of Z. schinifolium leaves; tBid, truncated Bid; COX VI, cytochrome oxidase subunit VI; MMP, mitochondrial membrane potential; MF, mitochondrial fraction; CF, cytosolic fraction; JC-1, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetramethyl-imidacarbocyanine iodide; DAPI, 4',6'-diamidino-2-phenylindole.
**P < 0.01 and ***P < 0.001 vs. untreated cells.
|
EEZS inactivates the PI3K/Akt signaling pathway in T24 cells
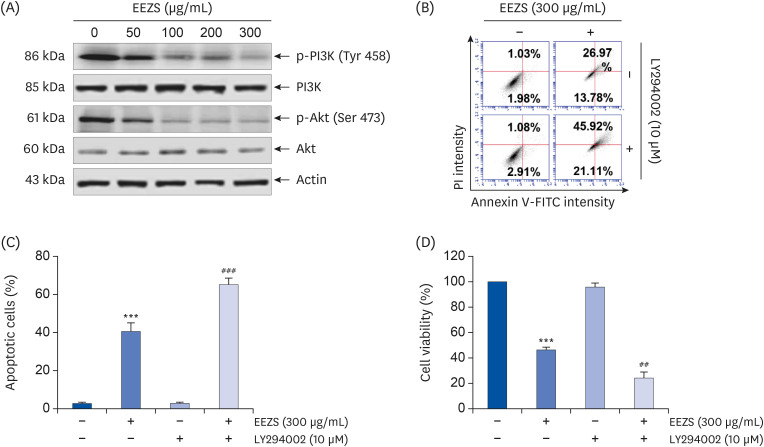 | Fig. 5Effect of EEZS in the PI3K/Akt signaling pathway in T24 cells. Cells were cultured for 48 h in medium containing the indicated concentrations of EEZS (A) or pre-treated with 10 μM LY294002 for 1 h, and then treated with 300 μg/mL EEZS for 48 h (B-D). (A) The expressions of PI3K and Akt proteins and their phosphorylated forms were determined by Western blot analysis. (B) Apoptosis of T24 cells after EEZS treatment in the presence or absence of LY29400 was analyzed using flow cytometry by annexin V/PI double staining. (C) Statistical analysis of apoptotic cell proportion. The percentage of cells undergoing apoptosis is expressed as the percentage of the number of annexin V-positive cells using a flow cytometer. (D) Cell viability was measured by the MTT assay. (C and D) Data are expressed as the mean ± SD.EEZS, ethanol extracts of Z. schinifolium leaves; PI3K, phosphoinositide 3-kinase; FITC, fluorescein isothiocyanate; PI, propidium iodine; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide.
***P < 0.001 vs. untreated cells. ##P < 0.01 and ###P < 0.001 vs. 300 μg/mL EEZS-treated cells.
|
EEZS increases ROS production in T24 cells
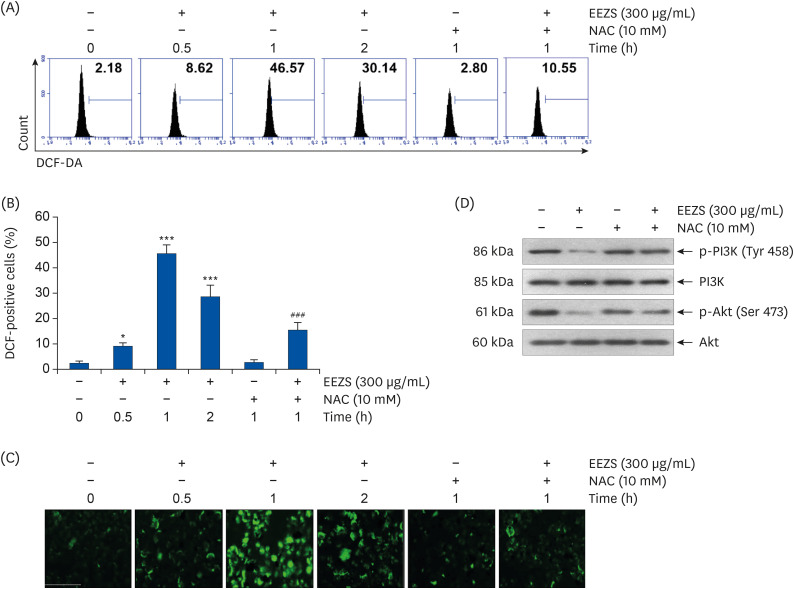 | Fig. 6Effect of ROS on inactivation of PI3K/Akt signaling pathway by EEZS in T24 cells. (A-C) Cells were treated with 300 μg/mL EEZS for the indicated periods, or pre-treated with or without 10 mM NAC for 1 h, followed by treatment with 300 μg/mL EEZS for 1 h. (A) ROS generation was detected by flow cytometry analysis. (B) Quantification of ROS generation measured by a flow cytometer. Data are expressed as the mean ± SD. (C) Intracellular ROS levels in T24 cells were detected using fluorescence microscopy. (D) Cells were incubated with 300 μg/mL EEZS for 48 h, or pre-incubated with 10 mM NAC for 1 h followed by treatment with 300 μg/mL EEZS for 48 h. The expressions of PI3K and Akt proteins and their phosphorylated forms were evaluated by Western blot analysis. Actin was used as a control for protein loading.EEZS, ethanol extracts of Z. schinifolium leaves; NAC; N-acetyl cysteine; DCF, 2′,7′-dichlorofluorescin; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species.
*P < 0.05 and ***P < 0.001 vs. untreated cells. ###P < 0.001 vs. 300 μg/mL EEZS-treated cells.
|
EEZS induces ROS-dependent apoptosis in T24 cells
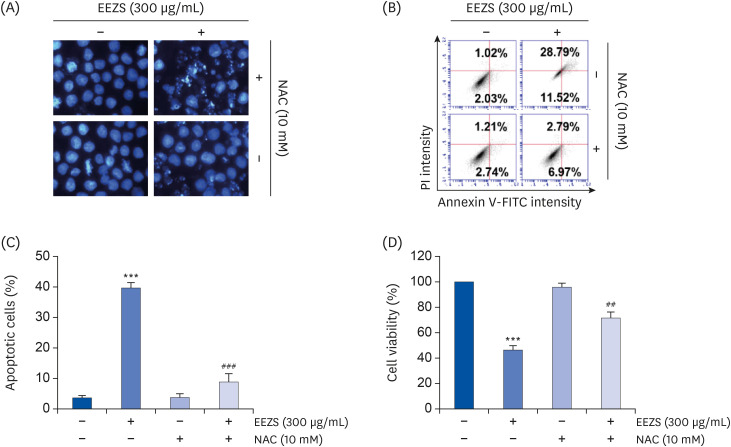 | Fig. 7Induction of ROS-dependent apoptosis by EEZS in T24 cells. Cells were treated with 300 μg/mL EEZS for 48 h, or pre-treated with 10 mM NAC for 1 h before 300 μg/mL EEZS treatment. (A) Nuclear morphological changes were observed under a fluorescence microscope using DAPI staining. (B) Apoptosis was analyzed using flow cytometry after annexin V/PI double staining in T24 cells. (C) Statistical analysis of apoptotic cell proportion. The percentage of cells undergoing apoptosis is expressed as the percentage of the number of annexin V-positive cells using a flow cytometer. (D) Cell viability was measured by the MTT assay. (C and D) Data are expressed as the mean ± SD.EEZS, ethanol extracts of Z. schinifolium leaves; NAC; N-acetyl cysteine; FITC, fluorescein isothiocyanate; PI, propidium iodine; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide; DAPI, 4′,6′-diamidino-2-phenylindole; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide.
***P < 0.001 vs. untreated cells. ##P < 0.01 and ###P < 0.001 vs. 300 μg/mL EEZS-treated cells.
|
DISCUSSION
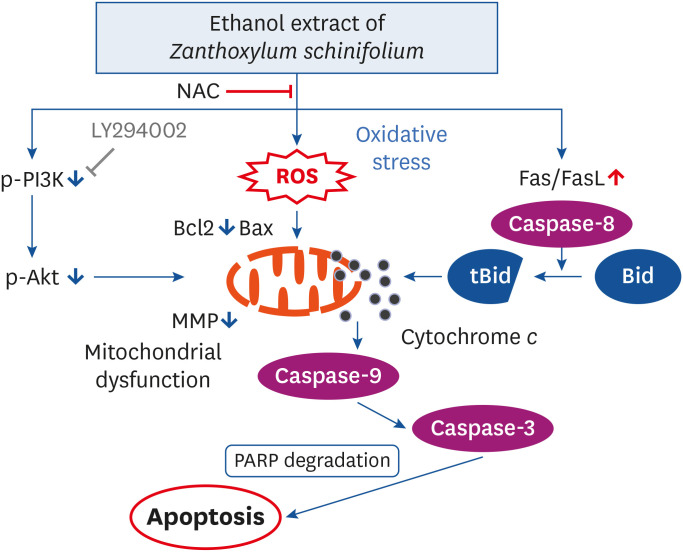 | Fig. 8Illustration of the mechanism of EEZS-induced apoptosis in human bladder cancer T24 cells.NAC, N-acetyl cysteine; ROS, reactive oxygen species; PI3K, phosphoinositide 3-kinase; FasL, Fas ligand; PARP, poly (ADP-ribose) polymerase; MMP, mitochondrial membrane potential; EEZS, ethanol extracts of Z. schinifolium leaves.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download