Abstract
Objective
Drug-eluting stents and balloons are occasionally used to reduce restenosis in medically intractable intracranial atherosclerotic stenosis. The authors aimed to determine whether such drugs can cause neurotoxicity due to local effects in a rat model.
Methods
Intra-arterial catheters were placed in the right common carotid artery of rats. Mannitol was injected to transiently open the brain-blood barrier (BBB), followed by high-dose drug (paclitaxel and rapamycin) injection. The optimal time interval of transient BBB opening for maximal drug penetration was determined to be 10 minutes. Paclitaxel and rapamycin were intraarterially administered in various doses. All the rats were neurologically evaluated, and their brain tissues were histologically examined.
Intracranial atherosclerotic stenosis (ICAS) is one of the causes of ischemic stroke. Recurrent stroke is known to occur in approximately 17% of patients with ICAS in 1 year, even with the best medical management [2,4]. Based on technical and clinical experience in the field of coronary artery diseases, endovascular treatment with stents has been attempted for patients with ICAS. Stenting in ICAS patients raises two major concerns : peri-procedural complications and high restenosis rates [2,4,8]. Regarding peri-procedural complications, recent studies have shown better outcomes than previous studies due to advancements in medical management, endovascular techniques and devices [2,4,8]. Regarding the issue of high restenosis rates after cerebral arterial stenting, a proper solution has not yet been identified. Drug-eluting stents (DESs) and drug-eluting balloons (DEBs) in the coronary system, which release drugs that inhibit intimal hyperplasia, have also been used for ICAS with good preliminary results in reducing restenosis [1,2,6,7,9,10,12,13,16]. However, applying these drugs to cerebral arteries may provoke neurotoxicity [2,5,12].
To our knowledge, no report has yet been published on this issue. The authors performed this study to evaluate neurotoxicity due to the local effects of high-dose paclitaxel and rapamycin using a rat model with transient blood-brain barrier (BBB) opening.
The procedures for handling and caring for animals adhered to the guidelines that are in compliance with current international laws and policies (National Institute of Health Guide for the Care and Use of Laboratory Animals, Publication No. 85-23, 1985, revised 1996), and they were approved by the Institutional Animal Care and Use Committee of Kangwon National University (KIACUC-12-0014).
Male, specific pathogen-free, Sprague Dawley rats were obtained from Bio-Genomics (Seoul, Korea). The rats were 7–8 months old and weighed 250–350 g. An intra-arterial infusion catheter was placed in the right common carotid artery.
To model the condition of a chronically ischemic brain with a partially disrupted BBB, temporary BBB opening was attempted via intra-arterial infusion of mannitol. A 20% mannitol solution (CJ Health Care, Daeso, Korea) was administered at a rate of 0.25 mL/kg/sec for 30 seconds with an infusion pump via the intra-arterial catheter. After mannitol infusion, a 2% solution of Evans Blue (EB; Sigma, St. Louis, MO, USA) in normal saline (4 mL/kg of body weight) was injected via the intra-arterial catheter to confirm the degree of BBB opening in predetermined time intervals. Twenty-four hours after EB infusion, the whole rat brain was harvested, and the degree of EB extravasation was evaluated. A total of 25 rats (five rats per predetermined interval) were used for this purpose. Based on the degree of EB extravasation, the optimal time interval for transient BBB opening and maximal drug delivery was determined to be 10 minutes (Figs. 1-3).
The doses of paclitaxel and rapamycin (Sigma) were translated from humans into animals as equivalent doses using a formula based on body surface area : animal equivalent dose (mg/kg) = human dose (mg/kg) × human Km / animal Km [14]. The drugs were injected as an intra-arterial bolus together with EB 10 minutes after mannitol administration. In the sham group, EB was infused without drugs. The brain tissues were obtained after 24 hours and 14 days. A total of 70 rats were used. Considering that the doses of the drugs coated on DESs and DEBs are 70–314 μg and 300–600 μg, respectively [18], a human dose of 600 μg was determined to be the maximum [2,4]. In this study, 1- to 4-times higher equivalent doses were tested.
Neurological status was evaluated using Bederson et al.[3]’s grading. Frozen rat brains fixed with paraformaldehyde were serially sectioned on a cryostat into 30-μm coronal sections. Histologic examination was performed with cresyl violet staining (Sigma) to assess neuronal death and Fluoro-Jade C (Histochem, Jefferson, AR, USA) histofluorescence staining to localize neuronal degeneration. Immunohistochemistry was performed with anti-glial fibrillary acidic protein (Biogenesis, San Ramon, CA, USA) for astrocytes and anti-ionized calcium-binding adapter molecule 1 (Wako, Osaka, Japan) for microglia. Detailed information is provided in the Supplement Method 1.
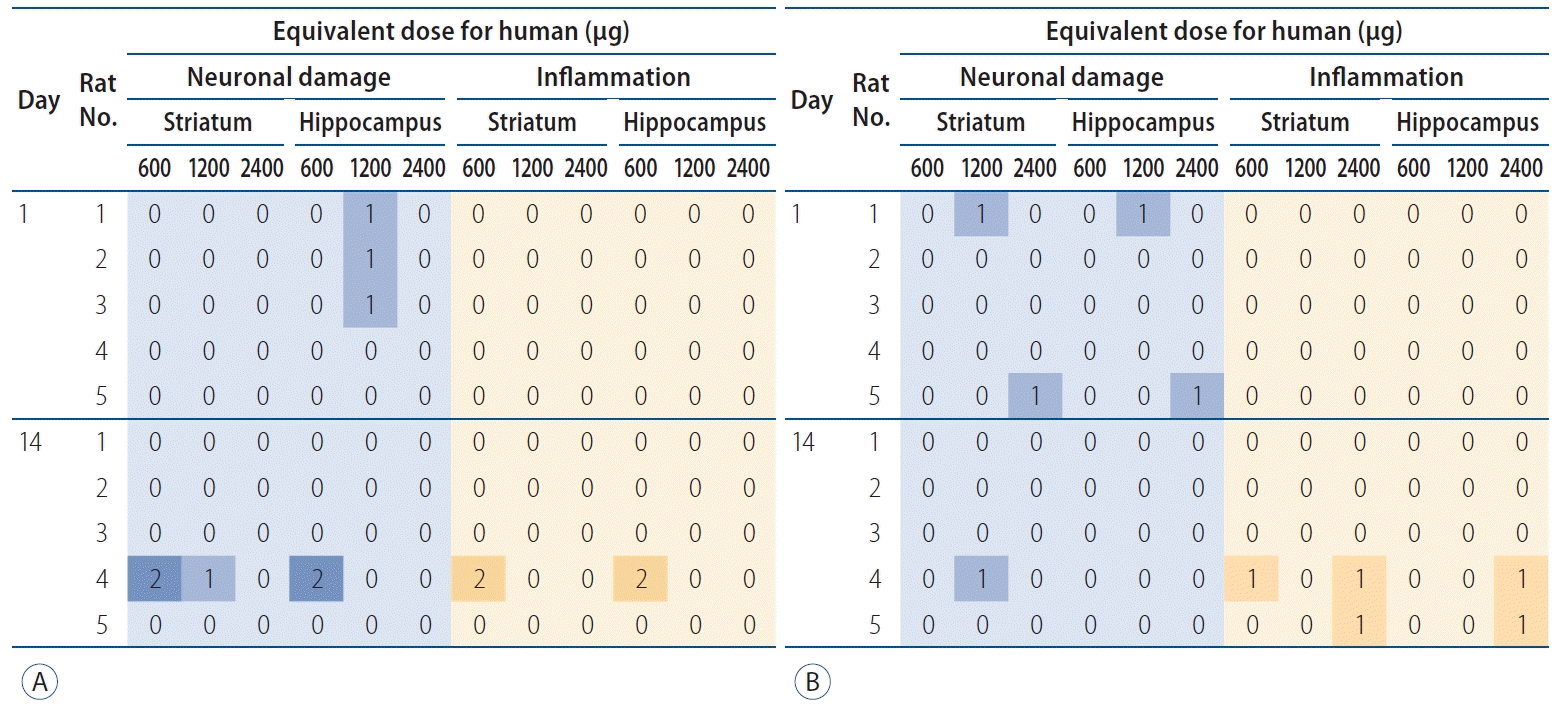
Most of rat brains were stained with EB, with a various range of grades from 1 to 3 (Supplementary Table 1). There were no histological abnormalities in the sham group (Supplementary Table 2, Supplementary Figs. 1 and 2). No neurotoxicity was observed (Bederson’s grade 0 in all cases) in any rats regardless of drug type, concentration or time period (Fig. 4 and Supplementary Figs. 3-14). Moderate degrees of inflammatory cell aggregation and neuronal damage were identified in 1 of 15 sections of just one rat brain (No. 4, day 14 after injection of 600 μg human paclitaxel dose; Supplementary Figs. 15 and 16). No rat showed neurological abnormalities.
The restenosis rate after treating symptomatic ICAS with bare-metal stents is known to be as high as approximately 30% [8,12]. Moreover, DESs and DEBs for ICAS caused lower rates of restenosis, ranging from 0% to 5% and from 0% to 13%, respectively [2,12]. Using DESs and DEBs in cerebral arteries could provoke neurotoxicity. Fortunately, chronic exposure of the brain to low doses of the drugs released from DEBs and DESs appears safe, based on the lack of reports about neurotoxicity due to systemic effects of the drugs in interventional cardiology over 15 years. And, not a few preliminary preclinical and clinical studies showed no local and systemic complications, yet [1,6,7,9,10,12,13,16]. In the early period after DES and DEB placement, high amounts of the drugs are released from the devices within the cerebral arteries. These high amounts could have harmful local effects on the brain. For example, 75–80% of the drugs are released into the bloodstream after DEB inflation, and only 10–15% are attached to the target vessel wall [18]. This study is the first animal experiment examining neurotoxicity due to the local effects of intra-arterial injection of high-dose paclitaxel and rapamycin. Our experiment showed no neurotoxicity, even though the drugs more easily penetrated into the brain tissues due to mannitol-induced BBB opening and higher doses, up to 4-times higher than the general dose on devices, were administered.
The major adverse event related to paclitaxel is peripheral neuropathy [17]. It has been reported that peripheral neuropathy of any grade can occur with 100 to 300 mg/m2 paclitaxel. The cumulative dose that causes severe neuropathy is known to be approximately 1000 mg/m2 [17]. For example, considering the surface dose (3 μg/mm2) and specifications of SeQuent® Please NEO (B. Braun Medical, Melsungen AG, Berlin, Germany), a novel paclitaxel-coated balloon ranging from 2.0×10 mm to 4.0×40 mm, the total surface dose is approximately 380 μg–3 mg. As the total surface dose ranges between 1/180–1/60 of the dose that causes peripheral neuropathy, paclitaxelrelated neurotoxicity hardly seems to occur, even if a full dose is released from the highest specification of the balloon. Moreover, paclitaxel cannot normally cross the BBB, and only two cases of grand mal seizure, leading to suspicion of central nervous system toxicity, have been reported [15]. Each ovarian cancer patient experienced seizures after intravenous injection of more than 250 mg/m2 paclitaxel in early clinical trials. However, the direct causality of paclitaxel is not clear because one patient had a brain metastasis and sub-therapeutic blood levels of phenytoin, and paclitaxel was not detected in the cerebrospinal fluid of the other patient.
Rapamycin is an immunosuppressant used for solid organ transplantation and a major drug coated on DESs. Rapamycin- related complications mainly include myelosuppression and hyperlipidemia. Although rapamycin easily crosses the BBB, neurotoxicity has not been reported except in five cases of posterior reversible encephalopathy, which was suspected to be caused by rapamycin-related neurotoxicity simply based on recovery after rapamycin discontinuation [5,11]. Therefore, even though our experiment did not show high-dose rapamycininduced neurotoxicity in a rat model, using rapamycin-coated devices needs to be avoided until the adverse effects in humans are clearly shown.
There are a few limitations in this study. First, only two prototype of drugs for the DESs and DEBs were used. However, these data would be applicable because newer drugs are derivatives of rapamycin and paclitaxel. Second, it is an animal experiment. Third, the degree of BBB opening in rat models was a little inhomogeneous, however, there was no tendency of the degree of BBB opening according to some factors. The authors did not attempt additional experiments, sacrificing more animals. Further experiments in vivo or in vitro under the control of the degree of BBB opening can be necessary. Fourth, rats’ neurological states were evaluated simply with a triage test which cannot detect minor changes.
There were no histological or neurological evidences of neurotoxicity by intra-arterial injection of paclitaxel and rapamycin in a rat model with transient BBB opening. This finding is expected to be helpful in consideration of the safety of drugs to reduce in-stent and post-procedural restenosis.
ACKNOWLEDGMENTS
This study was supported by a fund (02-2017-18) from Seoul National University Bundang Hospital.
Supplementary materials
The online-only data supplement is available with this article at https://doi.org/10.3340/jkns.2021.0077.
Supplementary Fig. 1.
Cresyl violet (A), glial fibrillary acidic protein (B), and ionized calcium-binding adapter molecule 1 (C) staining of No. 1 rat brain of sham group on the 1st day. There are no evidence of brain damage in the right hemispheres after transient blood-brain barrier opening with mannitol, compared to the normal left hemispheres. Scale bar, 2 mm.
Supplementary Fig. 2.
Cresyl violet (A), glial fibrillary acidic protein (B), and ionized calcium-binding adapter molecule 1 (C) staining of No. 1 rat brain of sham group on the 14th day. There are no evidence of brain damage in the right hemispheres after transient blood-brain barrier opening with mannitol, compared to the normal left hemispheres. Scale bar, 2 mm.
Supplementary Fig. 3.
Cresyl violet (A), glial fibrillary acidic protein (B), ionized calcium-binding adapter molecule 1 (C), and Fluoro-Jade C (D) staining of No. 1 rat brain on the 1st day after 600 μg human dose of paclitaxel. There are no evidence of brain damage in the right hemispheres after transient blood-brain barrier opening with mannitol, compared to the normal left hemispheres. Scale bar, 2 mm.
Supplementary Fig. 4.
Cresyl violet (A), glial fibrillary acidic protein (B), ionized calcium-binding adapter molecule 1 (C), and Fluoro-Jade C (D) staining of No. 1 rat brain on the 14th day after 600 μg human dose of paclitaxel. There are no evidence of brain damage in the right hemispheres after transient blood-brain barrier opening with mannitol, compared to the normal left hemispheres. Scale bar, 2 mm.
Supplementary Fig. 5.
Cresyl violet (A), glial fibrillary acidic protein (B), ionized calcium-binding adapter molecule 1 (C), and Fluoro-Jade C (D) staining of No. 4 rat brain on the 1st day after 1200 μg human dose of paclitaxel. There are no evidence of brain damage in the right hemispheres after transient blood-brain barrier opening with mannitol, compared to the normal left hemispheres. Scale bar, 2 mm.
Supplementary Fig. 6.
Cresyl violet (A), glial fibrillary acidic protein (B), ionized calcium-binding adapter molecule 1 (C), and Fluoro-Jade C (D) staining of No. 1 rat brain on the 14th day after 1200 μg human dose of paclitaxel. There are no evidence of brain damage in the right hemispheres after transient blood-brain barrier opening with mannitol, compared to the normal left hemispheres. Scale bar, 2 mm.
Supplementary Fig. 7.
Cresyl violet (A), glial fibrillary acidic protein (B), ionized calcium-binding adapter molecule 1 (C), and Fluoro-Jade C (D) staining of No. 1 rat brain on the 1st day after 2400 μg human dose of paclitaxel. There are no evidence of brain damage in the right hemispheres after transient blood-brain barrier opening with mannitol, compared to the normal left hemispheres. Scale bar, 2 mm.
Supplementary Fig. 8.
Cresyl violet (A), glial fibrillary acidic protein (B), ionized calcium-binding adapter molecule 1 (C), and Fluoro-Jade C (D) staining of No. 1 rat brain on the 14th day after 2400 μg human dose of paclitaxel. There are no evidence of brain damage in the right hemispheres after transient blood-brain barrier opening with mannitol, compared to the normal left hemispheres. Scale bar, 2 mm.
Supplementary Fig. 9.
Cresyl violet (A), glial fibrillary acidic protein (B), ionized calcium-binding adapter molecule 1 (C), and Fluoro-Jade C (D) staining of No. 1 rat brain on the 1st day after 600 μg human dose of rapamycin. There are no evidence of brain damage in the right hemispheres after transient blood-brain barrier opening with mannitol, compared to the normal left hemispheres. Scale bar, 2 mm.
Supplementary Fig. 10.
Cresyl violet (A), glial fibrillary acidic protein (B), ionized calcium-binding adapter molecule 1 (C), and Fluoro-Jade C (D) staining of No. 1 rat brain on the 14th day after 600 μg human dose of rapamycin. There are no evidence of brain damage in the right hemispheres after transient blood-brain barrier opening with mannitol, compared to the normal left hemispheres. Scale bar, 2 mm.
Supplementary Fig. 11.
Cresyl violet (A), glial fibrillary acidic protein (B), ionized calcium-binding adapter molecule 1 (C), and Fluoro-Jade C (D) staining of No. 2 rat brain on the 1st day after 1200 μg human dose of rapamycin. There are no evidence of brain damage in the right hemispheres after transient blood-brain barrier opening with mannitol, compared to the normal left hemispheres. Scale bar, 2 mm.
Supplementary Fig. 12.
Cresyl violet (A), glial fibrillary acidic protein (B), ionized calcium-binding adapter molecule 1 (C), and Fluoro-Jade C (D) staining of No. 1 rat brain on the 14th day after 1200 μg human dose of rapamycin. There are no evidence of brain damage in the right hemispheres after transient blood-brain barrier opening with mannitol, compared to the normal left hemispheres. Scale bar, 2 mm.
Supplementary Fig. 13.
Cresyl violet (A), glial fibrillary acidic protein (B), ionized calcium-binding adapter molecule 1 (C), and Fluoro-Jade C (D) staining of No. 1 rat brain on the 1st day after 2400 μg human dose of rapamycin. There are no evidence of brain damage in the right hemispheres after transient blood-brain barrier opening with mannitol, compared to the normal left hemispheres. Scale bar, 2 mm.
Supplementary Fig. 14.
Cresyl violet (A), glial fibrillary acidic protein (B), ionized calcium-binding adapter molecule 1 (C), and Fluoro-Jade C (D) staining of No. 1 rat brain on the 14th day after 2400 μg human dose of rapamycin. There are no evidence of brain damage in the right hemispheres after transient blood-brain barrier opening with mannitol, compared to the normal left hemispheres. Scale bar, 2 mm.
Supplementary Fig. 15.
Cresyl violet (A-F) and Fluoro-Jade C (G and H) staining of the No. 4 rat brain on 14th day after 600 μg human dose of paclitaxel. A and B (magnified photo of dotted circle in A), moderate aggregation of polymorphonuclear inflammatory cells in the striatum. C and D (magnified photo of dotted circle in C), frequent neuronal death in the cortex. E and F (magnified photo of dotted circle in E), frequent neuronal damage in the hippocampus. G and H (partly magnified photo of dotted circle in G), frequent neuronal degeneration in the striatum. Scale bar, 500 μm (E); 200 μm (A and G); 100 μm (B, C, F, and H); and 50 μm (D).
Supplementary Fig. 16.
Immunohistochemistry for glial fibrillary acidic protein (A-D) and ionized calcium-binding adapter molecule 1 (E and F) in the No. 4 rat brain on 14th day after 2400 μg human dose of rapamycin. A and B (magnified photo of dotted circle in A), rarely activated astrocytes in the striatum. C and D (magnified photo of dotted circle in C), rarely activated astrocytes in the cortex. E and F (magnified photo of dotted circle in E), rarely activated microglia in the cortex. Scale bar, 500 μm (A, C, and E); 100 μm (B); and 50 μm (D and F).
References
1. Abou-Chebl A, Bashir Q, Yadav JS. Drug-eluting stents for the treatment of intracranial atherosclerosis: initial experience and midterm angiographic follow-up. Stroke. 36:e165–e168. 2005.
2. Barnard ZR, Alexander MJ. Update in the treatment of intracranial atherosclerotic disease. Stroke Vasc Neurol. 5:59–64. 2019.

3. Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 17:472–476. 1986.

4. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 365:993–1003. 2011.
5. Gheith O, Cerna M, Halim MA, Nampoory N, Al-Otaibi T, Nair P, et al. Sirolimus-induced combined posterior reversible encephalopathy syndrome and lymphocytic pneumonitis in a renal transplant recipient: case report and review of the literature. Exp Clin Transplant. 15(Suppl 1):170–174. 2017.
6. Gupta R, Al-Ali F, Thomas AJ, Horowitz MB, Barrow T, Vora NA, et al. Safety, feasibility, and short-term follow-up of drug-eluting stent placement in the intracranial and extracranial circulation. Stroke. 37:2562–2566. 2006.

7. Kim J, Ban SP, Kim YD, Kwon OK. Long-term outcomes of drug-eluting stent implantation in patients with symptomatic extra- and intracranial atherosclerotic stenoses. J Cerebrovasc Endovasc Neurosurg. 22:216–224. 2020.

8. Kim SJ, Kim YJ, Ko JH. Long term outcome of in-stent stenosis after stent assisted coil embolization for cerebral aneurysm. J Korean Neurosurg Soc. 62:536–544. 2019.

9. Levy EI, Hanel RA, Howington JU, Nemes B, Boulos AS, Tio FO, et al. Sirolimus-eluting stents in the canine cerebral vasculature: a prospective, randomized, blinded assessment of safety and vessel response. J Neurosurg. 100:688–694. 2004.

10. Levy EI, Hanel RA, Tio FO, Garlick DS, Bailey L, Cunningham MR, et al. Safety and pharmacokinetics of sirolimus-eluting stents in the canine cerebral vasculature: 180 day assessment. Neurosurgery. 59:925–933. discussion 933-934. 2006.

11. Maramattom BV, Wijdicks EF. Sirolimus may not cause neurotoxicity in kidney and liver transplant recipients. Neurology. 63:1958–1959. 2004.

12. Park S, Lee DG, Chung WJ, Lee DH, Suh DC. Long-term outcomes of drug-eluting stents in symptomatic intracranial stenosis. Neurointervention. 8:9–14. 2013.

13. Qureshi AI, Kirmani JF, Hussein HM, Harris-Lane P, Divani AA, Suri MF, et al. Early and intermediate-term outcomes with drug-eluting stents in high-risk patients with symptomatic intracranial stenosis. Neurosurgery. 59:1044–1051. discussion 1051. 2006.

14. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 22:659–661. 2008.

15. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (taxol). Semin Oncol. 20(4 Suppl 3):1–15. 1993.
16. Steinfort B, Ng PP, Faulder K, Harrington T, Grinnell V, Sorby W, et al. Midterm outcomes of paclitaxel-eluting stents for the treatment of intracranial posterior circulation stenoses. J Neurosurg. 106:222–225. 2007.

18. Waksman R, Pakala R. Drug-eluting balloon: the comeback kid? Circ Cardiovasc Interv. 2:352–358. 2009.
Fig. 1.
A rat model for drug infusion via the right internal carotid artery with intra-arterial catheter indwelling.
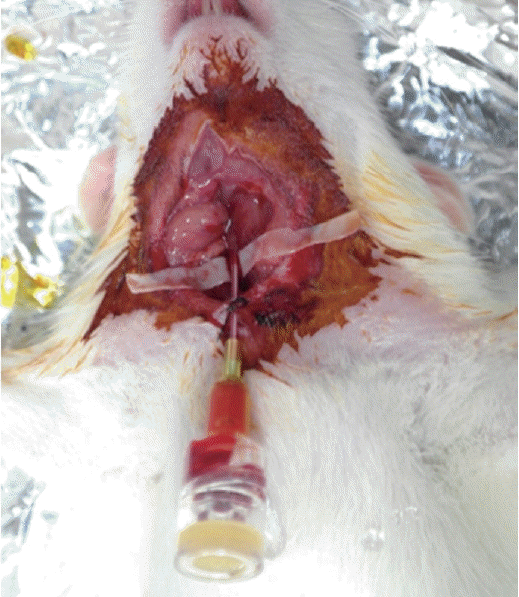
Fig. 2.
Degree of extravasation of Evans Blue in the right hemispheres of rat brains. A : Grade 0 when there is no evidence of extravasation. B : Grade 1 when the degree of extravasation is pale. C : Grade 2 when the degree is moderate. D : Grade 3 when the degree is strong.
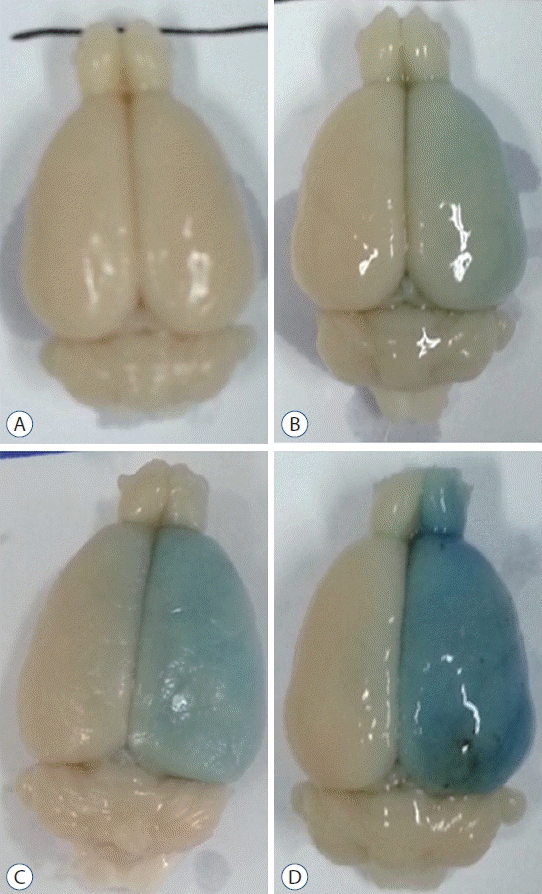
Fig. 3.
Optimal time of blood-brain barrier opening after mannitol infusion. The degree of Evans Blue extravasation is colored from faint blue as grade 0 to dark blue as grade 3.
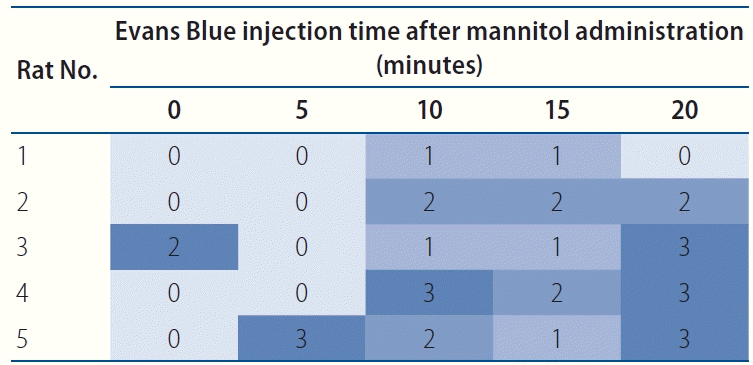
Fig. 4.
Histological change by paclitaxel (A) and rapamycin (B). The degree of neuronal damage and inflammation is colored from faint blue and orange each as grade 0 to dark blue and orange each as grade 3. All the positive histological findings are observed only on 1 or 2 of 15 slices of each right hemisphere.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download