1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019; 139:e56–528.
2. Lee SE, Lee HY, Cho HJ, Choe WS, Kim H, Choi JO, et al. Clinical characteristics and outcome of acute heart failure in Korea: results from the Korean Acute Heart Failure Registry (KorAHF). Korean Circ J. 2017; 47:341–53.

3. Ahn MS, Yoo BS, Yoon J, Lee SH, Kim JY, Ahn SG, et al. Prognostic effect of guideline-directed therapy is more noticeable early in the course of heart failure. J Korean Med Sci. 2019; 34:e133.

4. Kim MS, Lee JH, Kim EJ, Park DG, Park SJ, Park JJ, et al. Korean Guidelines for Diagnosis and Management of Chronic Heart Failure. Korean Circ J. 2017; 47:555–643.

5. Lee JH, Kim MS, Yoo BS, Park SJ, Park JJ, Shin MS, et al. KSHF Guidelines for the Management of Acute Heart Failure: Part II. Treatment of acute heart failure. Korean Circ J. 2019; 49:22–45.

6. Lee JH, Kim MS, Kim EJ, Park DG, Cho HJ, Yoo BS, et al. KSHF Guidelines for the Management of Acute Heart Failure: Part I. Definition, epidemiology and diagnosis of acute heart failure. Korean Circ J. 2019; 49:1–21.

7. Cryer PE, Wortsman J, Shah SD, Nowak RM, Deftos LJ. Plasma chromogranin A as a marker of sympathochromaffin activity in humans. Am J Physiol. 1991; 260(2 Pt 1):E243–6.

8. Eriksson B, Arnberg H, Oberg K, Hellman U, Lundqvist G, Wernstedt C, et al. Chromogranins: new sensitive markers for neuroendocrine tumors. Acta Oncol. 1989; 28:325–9.
9. Jansson AM, Røsjø H, Omland T, Karlsson T, Hartford M, Flyvbjerg A, et al. Prognostic value of circulating chromogranin A levels in acute coronary syndromes. Eur Heart J. 2009; 30:25–32.

10. Ceconi C, Ferrari R, Bachetti T, Opasich C, Volterrani M, Colombo B, et al. Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. Eur Heart J. 2002; 23:967–74.

11. Ottesen AH, Carlson CR, Louch WE, Dahl MB, Sandbu RA, Johansen RF, et al. Glycosylated chromogranin A in heart failure: implications for processing and cardiomyocyte calcium homeostasis. Circ Heart Fail. 2017; 10:e003675.
12. Estensen ME, Hognestad A, Syversen U, Squire I, Ng L, Kjekshus J, et al. Prognostic value of plasma chromogranin A levels in patients with complicated myocardial infarction. Am Heart J. 2006; 152:927.

13. Røsjø H, Masson S, Latini R, Flyvbjerg A, Milani V, La Rovere MT, et al. Prognostic value of chromogranin A in chronic heart failure: data from the GISSI-Heart Failure trial. Eur J Heart Fail. 2010; 12:549–56.

14. Kantar M, Levent E, Cetingul N, Ulger Z, Ozyurek R, Aksoylar S, et al. Plasma natriuretic peptides levels and echocardiographic findings in late subclinical anthracycline toxicity. Pediatr Hematol Oncol. 2008; 25:723–33.

15. Bhalla V, Willis S, Maisel AS. B-type natriuretic peptide: the level and the drug: partners in the diagnosis of congestive heart failure. Congest Heart Fail. 2004; 10(1 Suppl 1):3–27.

16. Huang B, Shen J, Li L, Huang Y, Luo S. Effect of B-type natriuretic peptide level on long-term outcome in patients with end-stage heart failure. Am J Cardiol. 2016; 118:383–8.

17. Kim MS, Lee JH, Cho HJ, Cho JY, Choi JO, Hwang KK, et al. KSHF Guidelines for the Management of Acute Heart Failure: Part III. Specific management of acute heart failure according to the etiology and co-morbidity. Korean Circ J. 2019; 49:46–68.

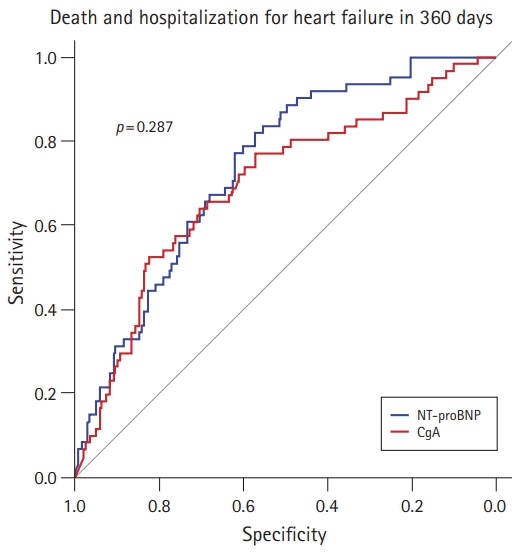
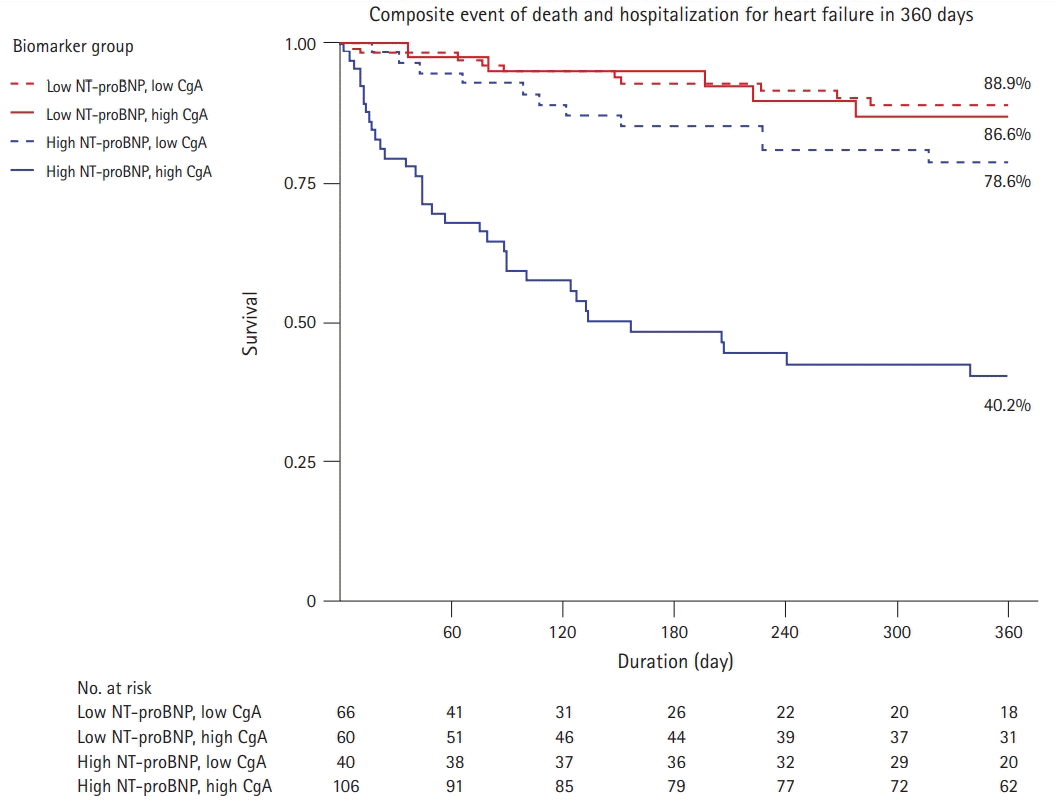
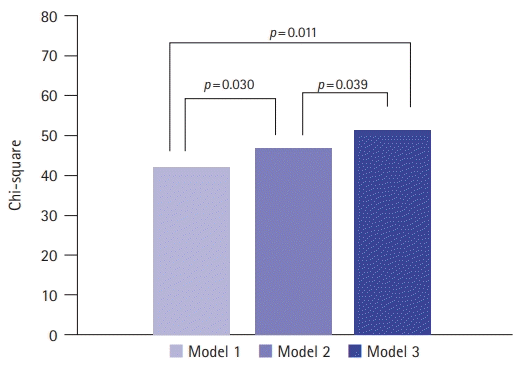




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download