1. Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015; 35. pii: e00191. DOI:
10.1042/BSR20150025. PMID:
25797907. PMCID:
4413017.

2. Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004; 18:980–982. DOI:
10.1096/fj.03-1100fje. PMID:
15084518.

3. Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, Tse HF, Fu QL, Lian Q. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016; 7:e2062. DOI:
10.1038/cddis.2015.327. PMID:
26794657. PMCID:
4816164.

4. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317. DOI:
10.1080/14653240600855905. PMID:
16923606.

5. Suila H, Pitkänen V, Hirvonen T, Heiskanen A, Anderson H, Laitinen A, Natunen S, Miller-Podraza H, Satomaa T, Natunen J, Laitinen S, Valmu L. Are globoseries glycosphingolipids SSEA-3 and -4 markers for stem cells derived from human umbilical cord blood? J Mol Cell Biol. 2011; 3:99–107. DOI:
10.1093/jmcb/mjq041. PMID:
21149348.

6. Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J, Prockop DJ. The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood. 2009; 113:816–826. DOI:
10.1182/blood-2007-12-128702. PMID:
18818395. PMCID:
2630267.

7. Pham H, Tonai R, Wu M, Birtolo C, Chen M. CD73, CD90, CD105 and Cadherin-11 RT-PCR screening for mesenchymal stem cells from cryopreserved human cord tissue. Int J Stem Cells. 2018; 11:26–38. DOI:
10.15283/ijsc17015. PMID:
29843192. PMCID:
5984056.

8. Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014; 32:1408–1419. DOI:
10.1002/stem.1681. PMID:
24578244.

10. Mahara A, Yamaoka T. Continuous separation of cells of high osteoblastic differentiation potential from mesenchymal stem cells on an antibody-immobilized column. Biomaterials. 2010; 31:4231–4237. DOI:
10.1016/j.biomaterials.2010.01.126. PMID:
20185169.

11. Chen AK, Reuveny S, Oh SK. Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: achievements and future direction. Biotechnol Adv. 2013; 31:1032–1046. DOI:
10.1016/j.biotechadv.2013.03.006. PMID:
23531528.

12. Sambu S, Xu X, Schiffer HA, Cui ZF, Ye H. RGDS-fuctionalized alginates improve the survival rate of encapsulated embryonic stem cells during cryopreservation. Cryo Letters. 2011; 32:389–401. PMID:
22020461.
13. Lee J, Abdeen AA, Kilian KA. Rewiring mesenchymal stem cell lineage specification by switching the biophysical microenvironment. Sci Rep. 2014; 4:5188. DOI:
10.1038/srep05188. PMID:
24898422. PMCID:
4046125.

14. Choi YS, Park SN, Suh H. Adipose tissue engineering using mesenchymal stem cells attached to injectable PLGA spheres. Biomaterials. 2005; 26:5855–5863. DOI:
10.1016/j.biomaterials.2005.02.022. PMID:
15949551.

15. Dalby MJ, Gadegaard N, Oreffo RO. Harnessing nano-topography and integrin-matrix interactions to influence stem cell fate. Nat Mater. 2014; 13:558–569. DOI:
10.1038/nmat3980. PMID:
24845995.

16. Walters NJ, Gentleman E. Evolving insights in cell-matrix interactions: elucidating how non-soluble properties of the extracellular niche direct stem cell fate. Acta Biomater. 2015; 11:3–16. DOI:
10.1016/j.actbio.2014.09.038. PMID:
25266503. PMCID:
5833939.

18. McMurray RJ, Gadegaard N, Tsimbouri PM, Burgess KV, McNamara LE, Tare R, Murawski K, Kingham E, Oreffo RO, Dalby MJ. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater. 2011; 10:637–644. DOI:
10.1038/nmat3058. PMID:
21765399.

19. Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CD, Oreffo RO. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007; 6:997–1003. DOI:
10.1038/nmat2013. PMID:
17891143.

20. Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater. 2008; 7:816–823. DOI:
10.1038/nmat2269. PMID:
18724374. PMCID:
2929915.

21. Phillips JE, Petrie TA, Creighton FP, García AJ. Human mesenchymal stem cell differentiation on self-assembled monolayers presenting different surface chemistries. Acta Biomater. 2010; 6:12–20. DOI:
10.1016/j.actbio.2009.07.023. PMID:
19632360. PMCID:
2787851.

22. Zamparelli A, Zini N, Cattini L, Spaletta G, Dallatana D, Bassi E, Barbaro F, Iafisco M, Mosca S, Parrilli A, Fini M, Giardino R, Sandri M, Sprio S, Tampieri A, Maraldi NM, Toni R. Growth on poly(L-lactic acid) porous scaffold preserves CD73 and CD90 immunophenotype markers of rat bone marrow mesenchymal stromal cells. J Mater Sci Mater Med. 2014; 25:2421–2436. DOI:
10.1007/s10856-014-5259-4. PMID:
24997163.

23. Duffy CR, Zhang R, How SE, Lilienkampf A, De Sousa PA, Bradley M. Long term mesenchymal stem cell culture on a defined synthetic substrate with enzyme free passaging. Biomaterials. 2014; 35:5998–6005. DOI:
10.1016/j.biomaterials.2014.04.013. PMID:
24780167.

24. Kasoju N, Wang H, Zhang B, George J, Gao S, Triffitt JT, Cui Z, Ye H. Transcriptomics of human multipotent mesenchymal stromal cells: retrospective analysis and future prospects. Biotechnol Adv. 2017; 35:407–418. DOI:
10.1016/j.biotechadv.2017.04.005. PMID:
28450077.

25. Sargent A, Shano G, Karl M, Garrison E, Miller C, Miller RH. Transcriptional profiling of mesenchymal stem cells identifies distinct neuroimmune pathways altered by CNS disease. Int J Stem Cells. 2018; 11:48–60. DOI:
10.15283/ijsc17062. PMID:
29699382. PMCID:
5984058.

26. Li Q, Zhang B, Kasoju N, Ma J, Yang A, Cui Z, Wang H, Ye H. Differential and interactive effects of substrate topography and chemistry on human mesenchymal stem cell gene expression. Int J Mol Sci. 2018; 19. pii: E2344. DOI:
10.3390/ijms19082344. PMID:
30096912. PMCID:
6121573.

27. Niehage C, Steenblock C, Pursche T, Bornhäuser M, Corbeil D, Hoflack B. The cell surface proteome of human mesenchymal stromal cells. PLoS One. 2011; 6:e20399. DOI:
10.1371/journal.pone.0020399. PMID:
21637820. PMCID:
3102717.

28. Cho KA, Park M, Kim YH, Woo SY, Ryu KH. RNA sequencing reveals a transcriptomic portrait of human mesenchymal stem cells from bone marrow, adipose tissue, and palatine tonsils. Sci Rep. 2017; 7:17114. DOI:
10.1038/s41598-017-16788-2. PMID:
29214990. PMCID:
5719355.

29. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of bio-molecular interaction networks. Genome Res. 2003; 13:2498–2504. DOI:
10.1101/gr.1239303. PMID:
14597658. PMCID:
403769.

30. Girvan M, Newman ME. Community structure in social and biological networks. Proc Natl Acad Sci U S A. 2002; 99:7821–7826. DOI:
10.1073/pnas.122653799. PMID:
12060727. PMCID:
122977.

32. Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 2014; 14:141–145. DOI:
10.1016/j.stem.2014.01.013. PMID:
24506881.

33. Skardal A, Mack D, Atala A, Soker S. Substrate elasticity controls cell proliferation, surface marker expression and motile phenotype in amniotic fluid-derived stem cells. J Mech Behav Biomed Mater. 2013; 17:307–316. DOI:
10.1016/j.jmbbm.2012.10.001. PMID:
23122714. PMCID:
3665276.

34. Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73). Purinergic Signal. 2006; 2:351–360. DOI:
10.1007/s11302-005-5302-5. PMID:
18404475. PMCID:
2254482.

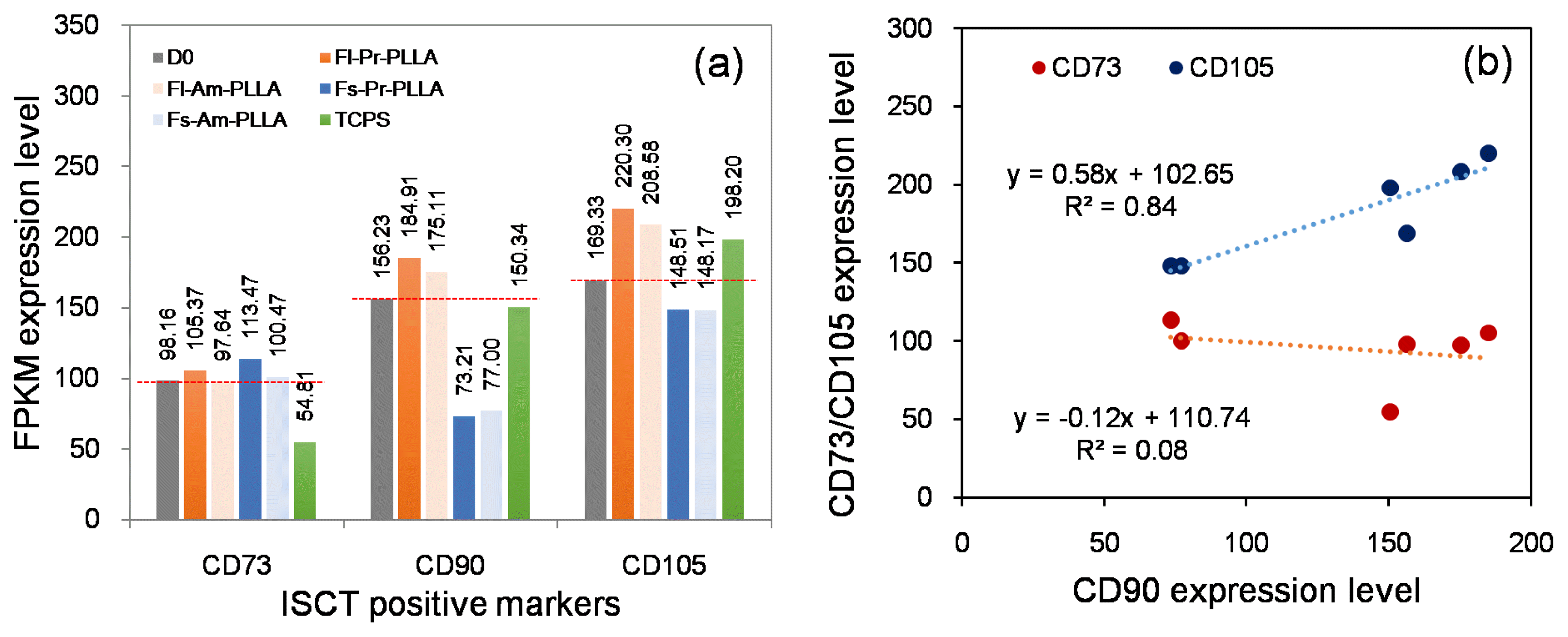
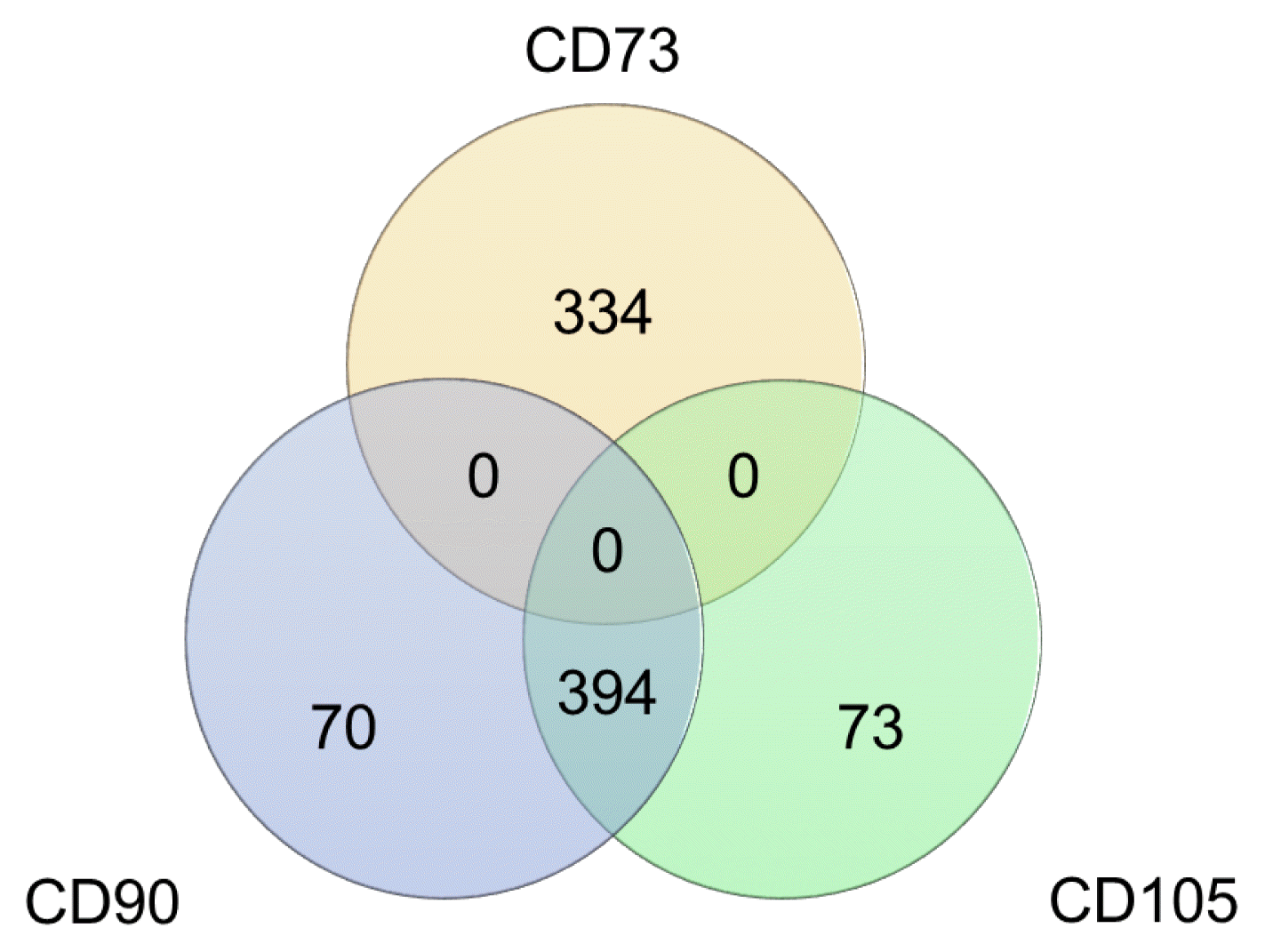
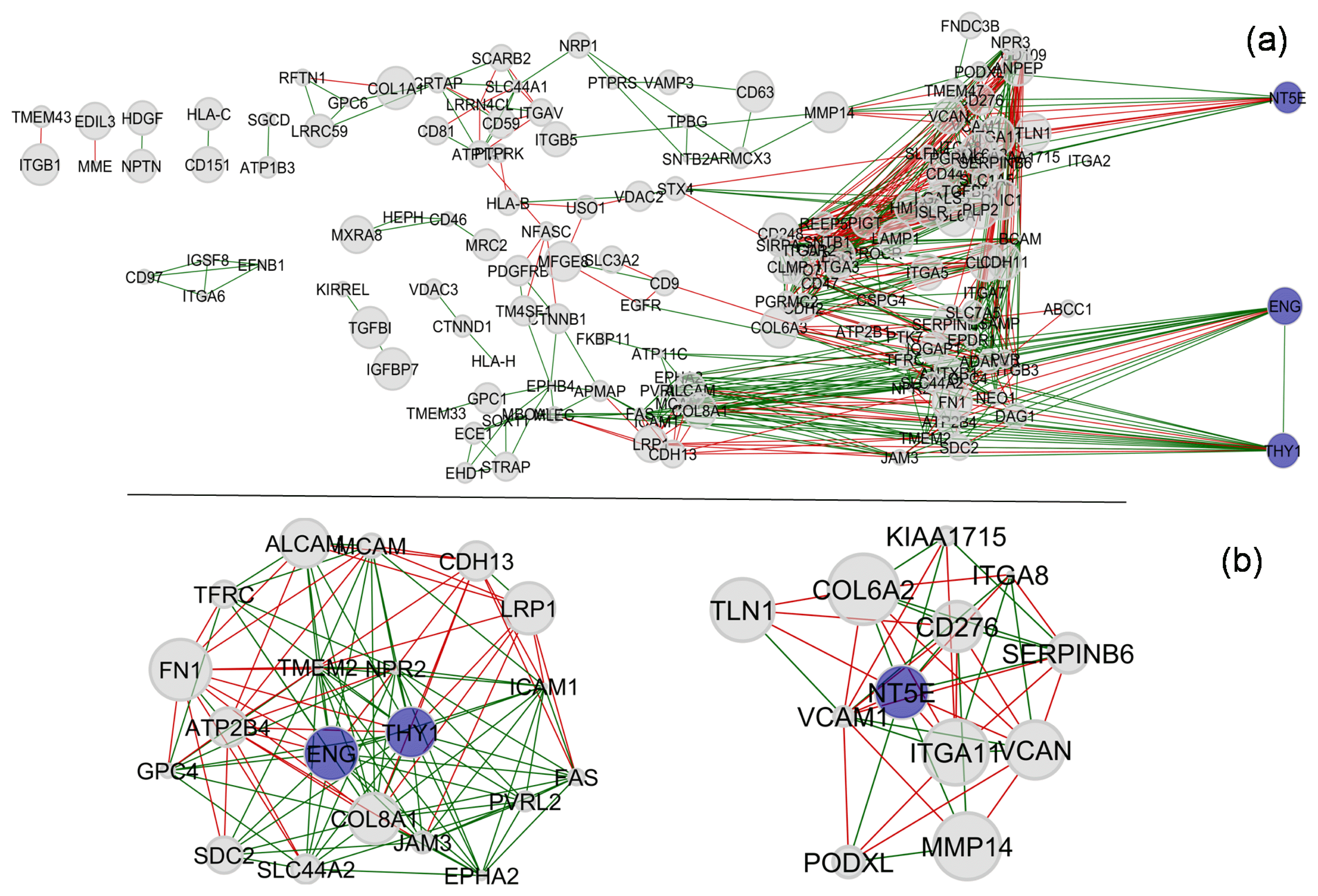
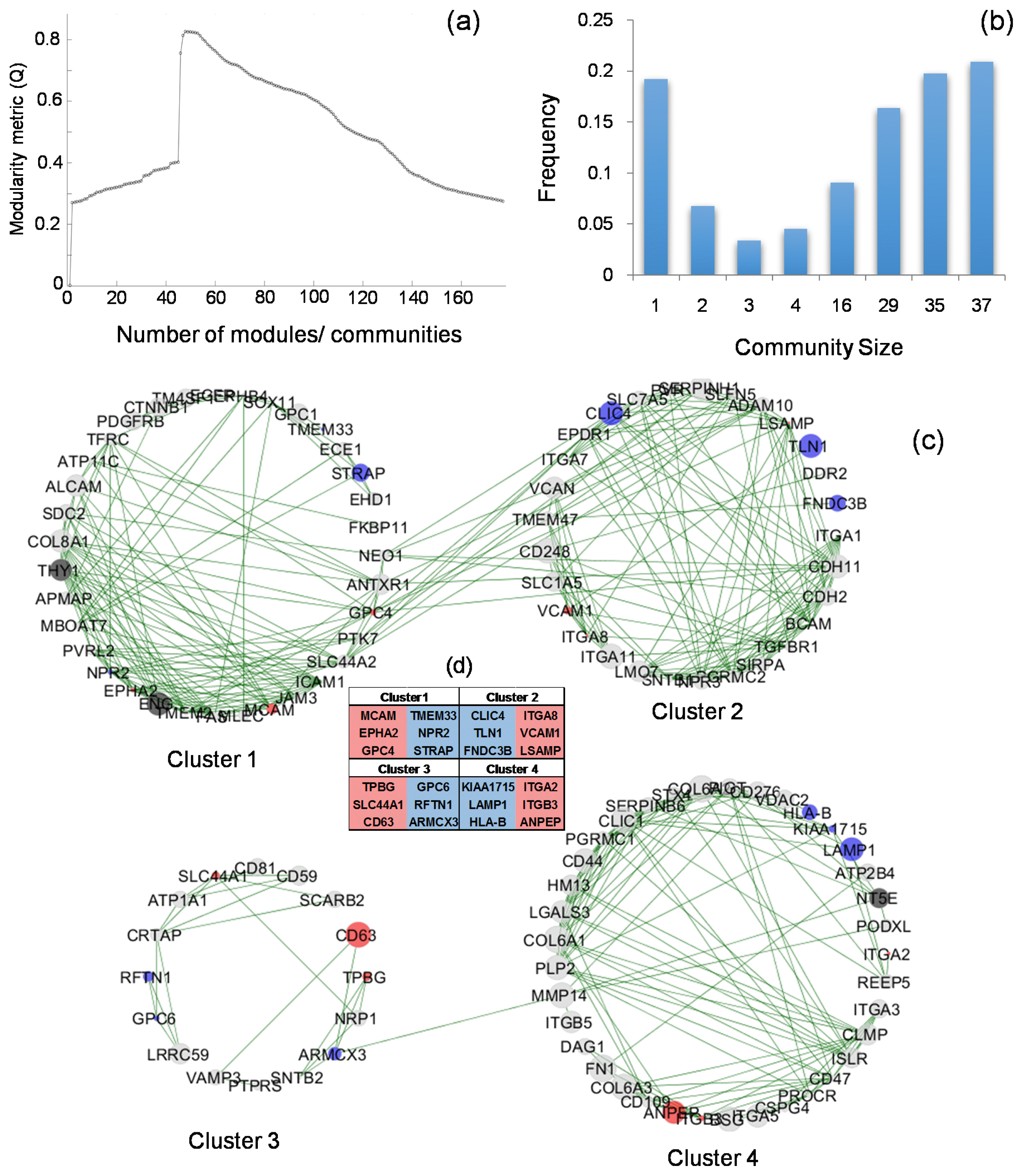




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download