Abstract
Purpose
Various hemostatic agents have been introduced in therapy as postoperative bleeding is a poor prognostic factor for postoperative outcomes. These products can be divided into those that directly promote the hemostatic cascade and those that physically form a barrier by absorbing blood. The latter, powder-type hemostatic agents have the advantages of being inexpensive and more absorbable with less foreign body reactions (FBRs) and are applicable to a relatively wide area. This study was conducted to verify the safety and efficacy of a newly invented polysaccharide product (OOZFIX, Theracion Biomedical), which improves blood absorption and hemostatic effects.
Methods
Two separate animal experiments were performed. The first evaluated FBRs histologically at 3 days, 2 weeks, and 4 weeks, after implantation of OOZFIX in rats, and the second compared hemostatic performance of OOZFIX and Arista AH (Bard) in the porcine liver punch biopsy model.
Results
We found minimal FBRs in the 3-day group and no reactions in both the 2-week and 4-week groups after implantation of hemostatic agents. The time to hemostasis of OOZFIX was not significantly different from that of Arista AH (median [interquartile range]: 9 [6–10] minutes vs. 8 [6–10] minutes, respectively; P = 0.522). When comparing the serial bleeding grade tendency, there was no statistical difference between OOZFIX and Arista AH (P = 0.656).
Perioperative or postoperative bleeding results in worse postoperative outcomes [123]. However, since most surgical procedures are inevitably accompanied by bleeding, various methods for hemostasis have been introduced, such as electrocauterization and compressive suture, among others [4]. However, these conventional hemostatic techniques cannot be used in some cases, especially for oozing without a clear source or bleeding around the major blood vessels.
Various commercially available products aid in achieving complete hemostasis upon application to the actively bleeding site [567]. These products can be broadly divided into 2 types according to their mechanisms of action. One is directly involved in the coagulation cascade, and the other physical forms a barrier to induce hemostasis. The former method is effective because it directly acts on the coagulation cascade, but it is expensive and uses a recombinant protein, and there is a concern about causing foreign body reactions (FBRs). The latter method includes powder-type hemostatic agents that comprise recently developed plant-derived polysaccharides or cellulose ingredients as Arista AH (Bard, Murray Hill, NJ, USA) and Surgicel powder (Johnson & Johnson, New Brunswick, NJ, USA). Since these products are composed of natural carbohydrates, FBRs are rare, the cost is low, and they can be applied to a relatively wide area.
A new powder product made of polysaccharides, OOZFIX (Theracion Biomedical, Seongnam, Korea), was developed to achieve better hemostatic capability (Supplementary Fig. 1). In this study, the hemostatic efficacy of the newly introduced product was compared with that of a commercially available product with the same mechanism and similar ingredients, Arista AH. The FBR was evaluated, both grossly and histopathologically after a certain period of time postimplantation in vivo. Most of the previously reported studies in this respect were company-driven studies, and may not have been free from conflicts of interest [8910]. This study was conducted to present results that were free from conflicting interests in powder-type hemostatic agents.
Before conducting experimental procedures, all experimental protocols were approved by the Animal Experimental Ethics Committee of the Korea Testing Laboratory (No. KTL-2020-H-0002-AP). All experiments were in compliance with the animal protection law (No. 16075, revised in 2018) as well as the laboratory animal law (No. 15944, revised in 2018), and all procedures were performed in accordance with the animal welfare requirements (ISO 10993-2, revised in 2006).
Eighteen male Sprague-Dawley species and specific-pathogen-free rats were purchased from Samtako Bio Korea (Osan, Korea). At the time of acquisition, the experimental animals were approximately 7 weeks old and weighed 219.4–234.6 g. The experiment was carried out after an acclimatization period of 5 days. All experimental animals were randomly assigned to 4 groups: 3 experimental groups and the control group. Five rats were placed in each experimental group, named 3D (autopsy 3 days after invagination), 2W (autopsy 2 weeks after invagination), and 4W (autopsy 4 weeks after invagination). Two rats were placed in the control group, C1 (autopsy 3 days after invagination).
The experimental animals were anesthetized using tiletamine + zolazepam 40 mg/kg, 0.8 mL/kg (Zoletil 50, Virbac Korea, Seoul, Korea) and xylazine 5 mg/kg, 0.2 mL/kg, intramuscular administration (Rompun 2%, Bayer Korea, Seoul, Korea). Among them, 2 subjects failed to achieve complete sedation despite the initial administration, as a result of which an additional 1/3 of the initial dosage was administered via the intramuscular route. Test and control substances were prepared for injection as follows; the test substance was prepared by filling 0.05 mL of OOZFIX into a 1-mL syringe in an aseptic environment. As a control material, high-density polyethylene was used, and it was prepared in a round cylindrical shape with an approximately 5-mm length and 2-mm diameter, and then autoclaved. The left flank of the anesthetized animal was widely draped with alcohol and povidone, and an incision of 3 mm or more was made. Subcutaneous pouches were made, on the cranial and caudal sides of the incision respectively (the distance from the incision line was more than 10 mm). The test substance was drawn into a 1-mL syringe, of which 0.05 mL was injected into each of the subcutaneous pouches. Similarly, subcutaneous pouches were made on the cranial and caudal sides of the incision site on the right flank. Two types of the control substance, 1 for each of the 2 pouches were similarly injected as described above. A total of 0.5 mL of the test substance (0.05 mL/site, 10 sites) and 10 control substances were invaginated into 5 experimental animals. After invagination, the incision was sutured using a nonabsorbable suture material (4-0 Ethilon, Ethicon, Somerville, NJ, USA). After the procedure was complete, the surgical site was marked using black ink. To determine the degree of damage caused by the surgical procedures, the procedure above was repeated in the control group, but no test or control materials were inserted.
After the invagination operation, the animals were carefully placed in a breeding box and monitored to see if they awoke from anesthesia normally. No antibiotics or analgesics were used, and the operation site was disinfected with gauze soaked in povidone-iodine solution. During the experimental period, the animals’ death and moribund statuses were checked at least once per day. Interval changes in the skin, hair, eyes, mucous membranes, respiratory system, circulatory system, autonomic/central nervous system, somatic motor system activities, and behavioral patterns were observed and recorded. After measuring the initial body weight of each animal, it was also measured at least once a week after the implantation and once before autopsy. When the study period had elapsed, all the animals were euthanized using CO2.
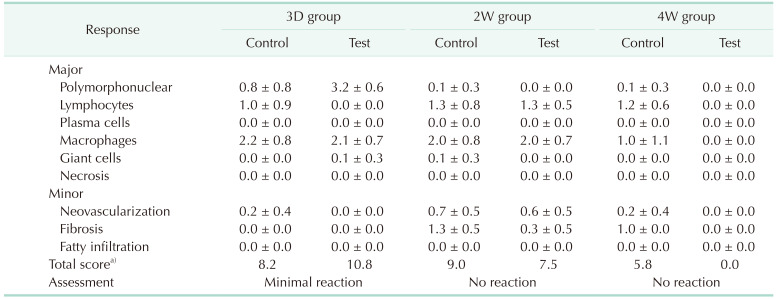
After confirming that the animals were euthanized, the tissues harvested from the invaginated site and the axillary lymph nodes on both sides were excised, the presence or absence of gross abnormalities (hematoma around the specimen, edema, encapsulation, etc.) was observed using a microscope, and photographs were taken. However, since the animals in the control group were used to determine the degree of tissue damage caused by invagination, gross observation of the axillary lymph nodes was not performed. After gross observation was completed for the remaining specimens, each was fixed in 10% neutral buffered formalin, and in the case of the control material, specimens were retrieved from the invaginated tissue 1 day after tissue fixation. Control materials (2 pieces per animal) invaginated to the right dorsal part of the experimental animals were retrieved. The properties, size, and shape of the implanted control substances were the same as those at the time of invagination, and all retrieved control substances were discarded. For all fixed tissues, histopathological examination was performed after staining with H&E. For implantation sites, 1 or more places were cut with a thickness of 2 mm or more in length or width of the specimen, including the site where the test or control substance was invaginated, and then embedded, sectioned, and stained. A photograph of the tissue (invaginated site) was taken for histopathological examination. For the histological observation of the transplant site, the number of polymorphonuclear lymphocytes, plasma cells, macrophages, and giant cells per high-power field (×400), and the degree of necrosis, neovascularization, fibrosis, and fatty infiltrate were evaluated by scoring (Supplementary Table 1). Based on the difference in evaluation score between the control and test substances, the types of reactions were classified into minimal or no reaction (0.0–2.9), slight reaction (3.0–8.9), moderate reaction (9.0–15.0), and severe reaction (>15.0).
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University Hospital (No. 20-02-03-S1A0), and animals were maintained in the facility accredited Association for Assessment and Accreditation of Laboratory Animal Care International (#001169) in accordance with the Guide for the Care and Use of Laboratory Animals 8th edition, National Research Council (2010). Prior to the experiment, all experimental procedures were approved by an accredited institution in accordance with the IACUC regulations. All researchers who participated in this experiment went through the certification process conducted by the IACUC and completed the workshop training for rodent experiments. To minimize pain in the study animals during the experimental procedures, all surgical procedures that may cause pain were performed after sufficient anesthesia. After completion of the experiments, the animals were euthanized under full sedation.
For the comparison of hemostatic performance, 3 Yorkshire pigs weighing approximately 60 kg were purchased from a specialized company (CRONEX, Hwaseong, Korea). The experiment was conducted after a stabilization period of 2 weeks in the breeding room of the Department of Experimental Animal Research, Biomedical Research Institute, Seoul National University Hospital. During the stabilization period, fresh fruits, vegetables, and feed for pigs were supplied, and the amount of drinking water was freely supplied by an individual automatic water supply system capable of disinfection (Guide for the Care and Use of Laboratory Animals, 8th edition in 2010).
On the day of the experiment, the pigs were placed in an operating room in a mobile cage. Then, the sedatives (Zoletil of 6 mg/kg + Rompun of 4.4 mg/kg) were injected intramuscularly. After sedation, animals were removed from the cage and placed in a prone position on the operation table, and the fluid was connected after obtaining an IV line in the ear. After putting a mask on and supplying 3%–5% isoflurane gas, the endotracheal tube was intubated using a laryngoscope. During the experiment, animals inhaled 1.5%–3% isoflurane gas through the endotracheal tube to maintain sufficient anesthesia.
After the experiment, euthanasia was performed by intravenous injection of a KCl solution under deep anesthesia. To prevent unexpected regeneration, the experiment was terminated after confirming cardiac arrest of the animal by flattening the electrocardiogram wave.
For this experiment, 5 OOZFIX was provided by Theracion Biomedical. Five Arista AH 2-g products were purchased for the control experiment. All products were sterilized using gamma-ray irradiation and stored at room temperature until the experiment. The double-packed product was opened and used immediately before the experiment. Since it was impossible to limit the quantity of powder required for each lesion, a sufficient quantity of each hemostatic agent was applied to cover the lesion completely.
Skin preparation and betadine draping were performed for the general anesthetized pigs. After applying the Mercedes incision using the No. 20 blade, an additional incision was made to the peritoneal layer to expose the entire liver. On the exposed liver surface, markings were made at intervals of approximately 3 cm with a marking pen. A disposable skin biopsy puncher (Paramount Surgimed, Delhi, India) was used to produce uniform bleeding lesions. To generate sufficient bleeding, punch biopsy was performed to a depth of 3 mm, and the remaining tissue pieces were removed using Metzenbaum scissors.
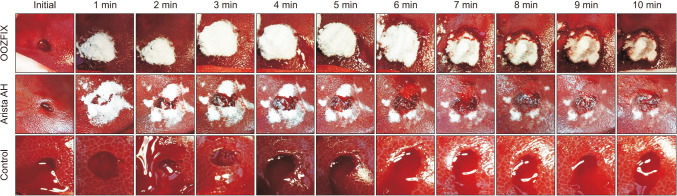
The previously published bleeding scale was designed for general bleeding, but this powder-type product was designed for milder bleeding [11]. Therefore, new criteria were devised and applied to this experiment with more subdivided criteria for grade 1 or 2 bleeding. Immediately after each lesion was produced, the initial status of bleeding from the lesion was evaluated. The grade of bleeding was visually checked by the observer and classified into 1 of 6 grades, which were defined as follows: grade 0, a state in which complete hemostasis was achieved; grade 1, oozing centered on the lesion that did not spill out from the lesion; grade 2, bleeding that did not exceed 50% of the lesion; grade 3, bleeding extended beyond the lesion but contained less than 50% of the border; grade 4, similar to grade 3, but above 50%; and grade 5, a condition in which ejection bleeding was observed from the lesion. Among these, lesions of grade 5 or higher were excluded from the experiment, and bleeding was stopped through electrocauterization. Since a blind test was not possible due to differences in product properties, after classifying each initial lesion, the hemostatic products, OOZFIX and Arista AH, were applied to each lesion of the test group. No product was applied to the control group. Since the median time to hemostasis of Arista AH was found to be 288 seconds in a previously published study [10], it was expected that complete hemostasis would occur 5 minutes after application of the product. After 5 minutes of product application for each lesion, the excessive product was removed by wet gauze. During the 10 minutes after applying the products, each lesion was photographed every minute. After the experiment, the photographs were evaluated according to the bleeding grade without knowing the type of applied product.
The primary endpoint of this experiment was the time to achieve complete hemostasis, if rebleeding occurs in a lesion that had shown complete hemostasis before 10 minutes, then the hemostasis time was recorded as the time when the rebleeding finally stopped. In addition, the rate of achieving hemostasis within 10 minutes, the rate of rebleeding after achieving complete hemostasis (grade 0), and the degree of bleeding at each time point were recorded.
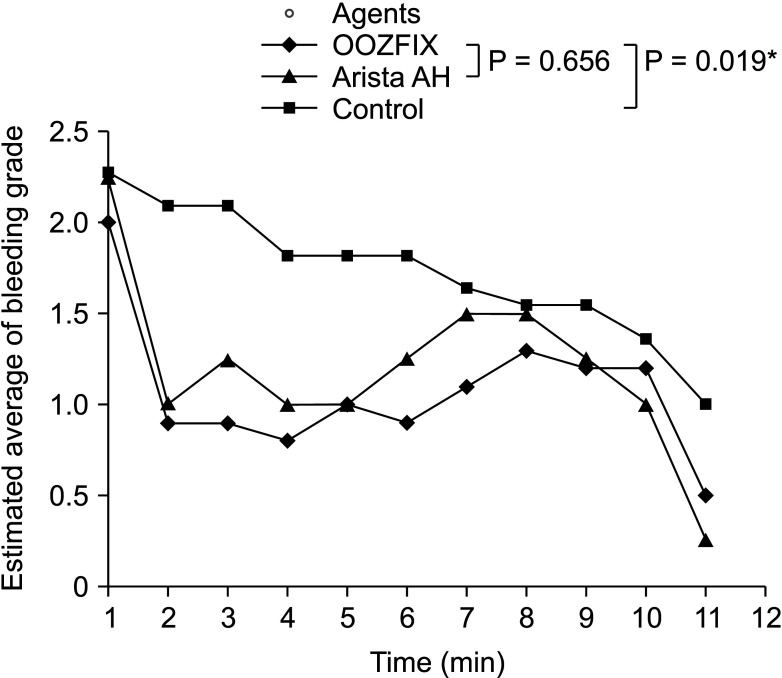
For statistical testing of the experimental results, IBM SPSS Statistics ver. 25 (IBM Corp., Armonk, NY, USA) was used. Since the bleeding grade used to measure the bleeding status was not a continuous variable, the chi-square test was used to compare the degree of initial bleeding. Analysis of covariance, a nonparametric test, was used to compare the time to achieve final hemostasis, and the Mann-Whitney U-test was performed between the 2 groups for post hoc analysis. Repeated measures analysis of variance (ANOVA) was used to compare the trends in the bleeding grade between the groups. As a result of the tests, a P-value of <0.05 was considered statistically significant.
There were no deaths or pathologic symptoms observed in any of the experimental animals during the study period. Temporary weight loss was observed in all groups due to recovery from surgery and fasting before autopsy, without any relationship to the implanted materials. During the experimental period, no implant-induced findings were observed at the implantation site or axillary lymph nodes of any of the test animals. In the 3D group, which was harvested on the 3rd day after implantation of the test substance, the shape of the test substance was not seen, but a seroma was observed in the subcutaneous tissue. No test substances or related abnormal findings were identified in the 2W or 4W groups.
Histopathological examination of the axillary lymph nodes revealed no abnormal findings related to the implanted products. The findings at the implantation site were generally not different between the test and control materials.
In the test group of 3D, 2W, and 4W, there were definite findings corresponding to the local inflammation where the control substance was implanted. In contrast, there were different findings where the test substance was implanted. In the 3D group, there were reactive findings due to inflammation similar to those seen in the control, including where the test substance was implanted. However, only granulation tissue was observed in the implanted space in the 2W group. Only normal tissue was found where the test substance was implanted in the 4W group. Based on the differences in histological evaluation scores between the test and control substances, the 3D group was classified as having minimal reaction, and both 2W and 4W groups were evaluated as having no reaction (Table 1, Fig. 1).
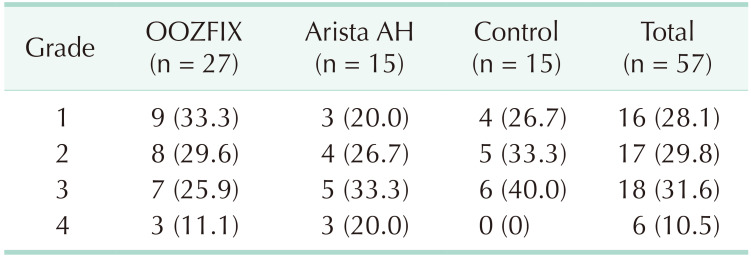
A total of 60 liver punch biopsy lesions were prepared from 3 experimental pigs. Of these, 30 lesions were in the OOZFIX group, another 15 lesions were in the Arista AH-applied group, and the other 15 lesions were in the control group, which were left untreated (Fig. 2). Of the 30 lesions in the OOZFIX group, 3 lesions were evaluated as bleeding grade 5 and excluded from further experiments. The distribution of the initial bleeding grades in each group is shown in Table 2. There was no statistical difference between the groups.
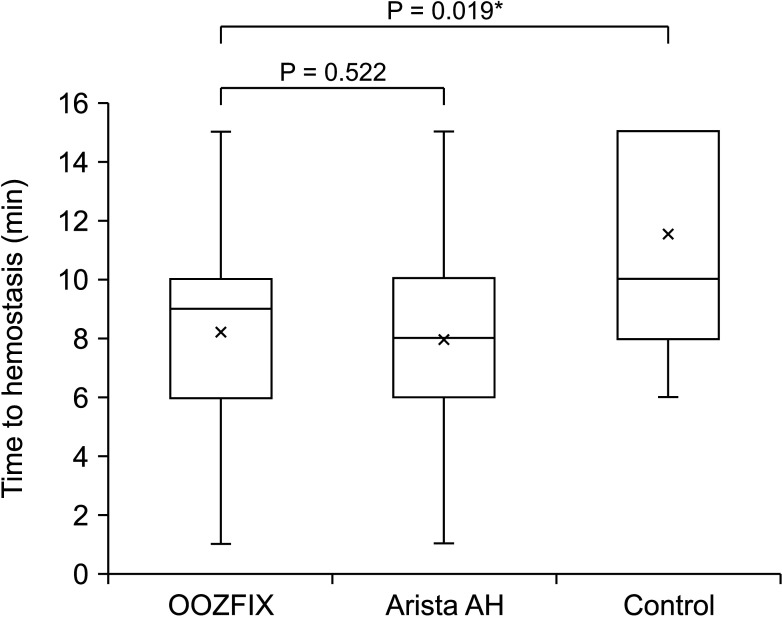
The time required for complete hemostasis was compared between groups (Fig. 3). In particular, lesions that failed to achieve complete hemostasis (grade 0) within 10 minutes were evaluated for 15 minutes. The OOZFIX group (median, 9 minutes; interquartile range [IQR], 6–10 minutes) did not show a statistically significant difference from the Arista AH group (median, 8 minutes; IQR, 6–10 minutes; P = 0.522), and showed a statistically significantly better result than the control group (median, 10 minutes; IQR, 8–15 minutes; P = 0.019). Using repeated measures ANOVA to compare the bleeding grade tendencies between the groups, we again found no statistical difference between the OOZFIX and Arista AH groups (P = 0.656), but there was a significant difference seen between the OOZFIX and control groups (P = 0.019) (Fig. 4).
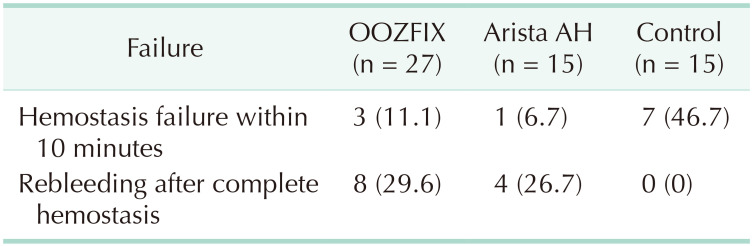
The number of cases in which hemostasis failed within 10 minutes in each group was 3 in the OOZFIX group (11.1%), 1 in the Arista AH group (6.7%), and 7 cases in the control group (46.7%). In particular, 8 cases of OOZFIX (29.6%) and 4 cases of Arista AH (26.7%) showed rebleeding during the 10 minutes of observation after achieving complete hemostasis (grade 0) (Table 3).
Since peri- or postoperative bleeding is one of the factors that directly affect postoperative prognosis, various methods have been tried to prevent it [4]. However, these methods have both advantages and disadvantages. In particular, in organs, such as the liver, spleen, and kidneys, which have abundant blood flow in the abdominal cavity, postoperative bleeding can have adverse effects. When bleeding is observed in these organs, it is difficult to accurately identify the bleeding point in most cases; therefore, it is difficult to adjust electrocauterization or apply patch-type hemostatic products. When the bleeding source is cauterized in such cases, there is a risk of inducing bleeding by damaging the major vessels located in deeper areas as a result of thermal injury. Following application of patch-type products if there is oozing, the product may be washed off and removed from the proper position.
To circumvent these problems, powder-based hemostatic agents have recently been introduced. These products absorb surrounding moisture and form a physical barrier called microporous polysaccharide hemospheres, so they can be effective during continuous oozing [10]. OOZFIX is a calcium-modified carboxyl starch with a higher calcium ion content compared to that in existing powder-type products. The hemostatic capability was designed to be enhanced by electrostatically attracting platelets and negatively charged coagulation proteins due to the increased calcium ion concentration. In addition, as it showed stronger hydrophilicity as measured by the contact angle, a further improved hemostatic effect can be expected. In this study, using the porcine liver punch biopsy model, the newly introduced product (OOZFIX) did not show a statistically significant difference in the hemostatic time and trends of bleeding grade compared to the previously commercialized product (Figs. 1, 2). Both products showed statistically better hemostatic performance than that observed in the control group without any manipulation.
In addition, since the components of thrombin-type hemostatic agents that act directly on the hemostatic cascade have bovine or humanized synthesis, there is a risk of complications by inducing an FBR in vivo [121314]. However, as the powder-type hemostatic product uses plant-based raw materials, the chance of inducing an FBR is reduced, and rapid biodegradation can be expected [1516]. In this study, an FBR was grossly identified in the 3D group, but granulation tissue was seen in the 2W group, and there was normal tissue in the 4W group. Therefore, it was quickly degraded in the body, and histopathological examination confirmed that the local FBR induced by the test substance was also insignificant.
A significant number of cases showed rebleeding, which occurred in 8 cases in the OOZFIX group (29.6%) and 4 cases in the Arista AH group (26.7%) (Table 2). In this case, bleeding was observed again after achieving complete hemostasis (evaluated as grade 0). As rebleeding occurred at a similar rate in both treated groups, it shows the limitations of powder-based hemostatic agents rather than limitations of a specific product. Due to the nature of the product, when a sufficient amount of the product is applied to each lesion, there is no way to visually check whether the hemostasis has been completed or is still bleeding from the outside. Therefore, when removing the excessive product, the possibility that the physical external stimulus affected the result cannot be excluded. Further research on the relationship between the amount of product applied and the hemostasis success rate will be needed. Rebleeding was observed in a significant number of lesions to which the powder products were applied, regardless of the excessive product removal process. Due to the nature of the powder formulation, if the adherence of the barrier physically formed on the bleeding surface is interrupted, bleeding can restart at any time. This hemostasis mechanism suggests that the stability of the surface on which the product is applied, regardless of the degree of bleeding, has a great influence on the success of permanent hemostasis. Therefore, it may be effective for hard surfaces, but for soft organs such as the liver or spleen, it would be desirable to apply a sufficient quantity of product with minimal manipulation after achieving initial hemostasis.
This study has several limitations. The powder formulation used in the experiment was visually distinguishable, making a double-blind experiment impossible. However, for each lesion, pictures were taken at intervals of 1 minute after application of the product, and the degree of bleeding was evaluated from the pictures taken; therefore, the evaluator was unaware of the product used.
In conclusion, this study confirmed that a new powder-type polysaccharide hemostatic agent effectively induced hemostasis in a porcine liver punch biopsy model. In an FBR experiment using a rat model, the powder product was visibly degraded within a relatively short time, and it rarely caused pathological FBRs. Future clinical studies can be planned through the proper use of the product.
References
1. Montán C, Wannberg M, Holst J, Wahlgren CM. Perioperative haemorrhage in endovascular abdominal aneurysm repair affects outcome. Eur J Vasc Endovasc Surg. 2013; 46:87–92. PMID: 23582344.

2. Hachem LD, Ghanekar A, Selzner M, Famure O, Li Y, Kim SJ. Postoperative surgical-site hemorrhage after kidney transplantation: incidence, risk factors, and outcomes. Transpl Int. 2017; 30:474–483. PMID: 28120465.

3. Schrem H, Klußmann A, Focken M, Emmanouilidis N, Oldhafer F, Klempnauer J, et al. Post-operative hemorrhage after liver transplantation: risk factors and long-term outcome. Ann Transplant. 2016; 21:46–55. PMID: 26818716.

4. Shah A, Palmer AJ, Klein AA. Strategies to minimize intraoperative blood loss during major surgery. Br J Surg. 2020; 107:e26–e38. PMID: 31903592.

5. Seyednejad H, Imani M, Jamieson T, Seifalian AM. Topical haemostatic agents. Br J Surg. 2008; 95:1197–1225. PMID: 18763249.

6. Boonstra EA, Molenaar IQ, Porte RJ, de Boer MT. Topical haemostatic agents in liver surgery: do we need them? HPB (Oxford). 2009; 11:306–310. PMID: 19718357.

7. Brustia R, Granger B, Scatton O. An update on topical haemostatic agents in liver surgery: systematic review and meta analysis. J Hepatobiliary Pancreat Sci. 2016; 23:609–621. PMID: 27580747.

8. Lewis KM, Atlee HD, Mannone AJ, Dwyer J, Lin L, Goppelt A, et al. Comparison of two gelatin and thrombin combination hemostats in a porcine liver abrasion model. J Invest Surg. 2013; 26:141–148. PMID: 23514063.

9. Singh RK, Baumgartner B, Mantei JR, Parreno RN, Sanders PJ, Lewis KM, et al. Hemostatic comparison of a polysaccharide powder and a gelatin powder. J Invest Surg. 2019; 32:393–401. PMID: 29420097.

10. MacDonald MH, Wang AY, Clymer JW, Hutchinson RW, Kocharian R. An in vivo comparison of the efficacy of hemostatic powders, using two porcine bleeding models. Med Devices (Auckl). 2017; 10:273–279. PMID: 29238233.
11. Lewis KM, Li Q, Jones DS, Corrales JD, Du H, Spiess PE, et al. Development and validation of an intraoperative bleeding severity scale for use in clinical studies of hemostatic agents. Surgery. 2017; 161:771–781. PMID: 27839931.

12. Rosselli DD, Brainard BM, Schmiedt CW. Efficacy of a topical bovine-derived thrombin solution as a hemostatic agent in a rodent model of hepatic injury. Can J Vet Res. 2015; 79:303–308. PMID: 26424911.
13. Franceschini G. Internal surgical use of biodegradable carbohydrate polymers. Warning for a conscious and proper use of oxidized regenerated cellulose. Carbohydr Polym. 2019; 216:213–216. PMID: 31047059.

14. Yang YL, Zhang C, Zhang HW, Wu P, Ma YF, Lin MJ, et al. Common bile duct injury by fibrin glue: report of a rare complication. World J Gastroenterol. 2015; 21:2854–2857. PMID: 25759561.

15. Antisdel JL, Janney CG, Long JP, Sindwani R. Hemostat ic agent microporous polysaccharide hemospheres (MPH) does not affect healing or intact sinus mucosa. Laryngoscope. 2008; 118:1265–1269. PMID: 18438268.

16. Ereth MH, Schaff M, Ericson EF, Wetjen NM, Nuttall GA, Oliver WC Jr. Comparative safety and efficacy of topical hemostatic agents in a rat neurosurgical model. Neurosurgery. 2008; 63(4 Suppl 2):369–372. PMID: 18981845.

SUPPLEMENTARY MATERIALS
Supplementary Table 1 and Supplementary Fig. 1 can be found via https://doi.org/10.4174/astr.2022.102.2.65.
Supplementary Table 1
Criteria for evaluating the degree of histological response
Supplementary Fig. 1
Scanning electron microscopic images for comparison of particle surface of (A) OOZFIX (Theracion Biomedical, Seongnam, Korea) and (B) Arista AH (Bard, Murray Hill, NJ, USA).




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download