1. Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest. 2005; Mar. 127(3):984–1005.
2. Boogaard R, Huijsmans SH, Pijnenburg MW, Tiddens HA, de Jongste JC, Merkus PJ. Tracheomalacia and bronchomalacia in children: incidence and patient characteristics. Chest. 2005; Nov. 128(5):3391–7.
3. McNamara VM, Crabbe DC. Tracheomalacia. Paediatr Respir Rev. 2004; Jun. 5(2):147–54.

4. Saito Y, Imamura H. Airway stenting. Surg Today. 2005; 35(4):265–70.

5. Zakaluzny SA, Lane JD, Mair EA. Complications of tracheobronchial airway stents. Otolaryngol Head Neck Surg. 2003; Apr. 128(4):478–88.

6. Korraa E, Madkour A, Todary A, Wagieh K. Evaluation of bronchoscopic placement of tracheobronchial silicone stents: an Ain Shams University Hospital experience. Egypt J Bronchol. 2014; 8(1):38–43.

7. Jacobs JP, Quintessenza JA, Botero LM, van Gelder HM, Giroud JM, Elliott MJ, et al. The role of airway stents in the management of pediatric tracheal, carinal, and bronchial disease. Eur J Cardiothorac Surg. 2000; Nov. 18(5):505–12.

8. Saad CP, Murthy S, Krizmanich G, Mehta AC. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest. 2003; Nov. 124(5):1993–9.
9. Lunn W, Feller-Kopman D, Wahidi M, Ashiku S, Thurer R, Ernst A. Endoscopic removal of metallic airway stents. Chest. 2005; Jun. 127(6):2106–12.

10. Geller KA, Wells WJ, Koempel JA, St John MA. Use of the Palmaz stent in the treatment of severe tracheomalacia. Ann Otol Rhinol Laryngol. 2004; Aug. 113(8):641–7.

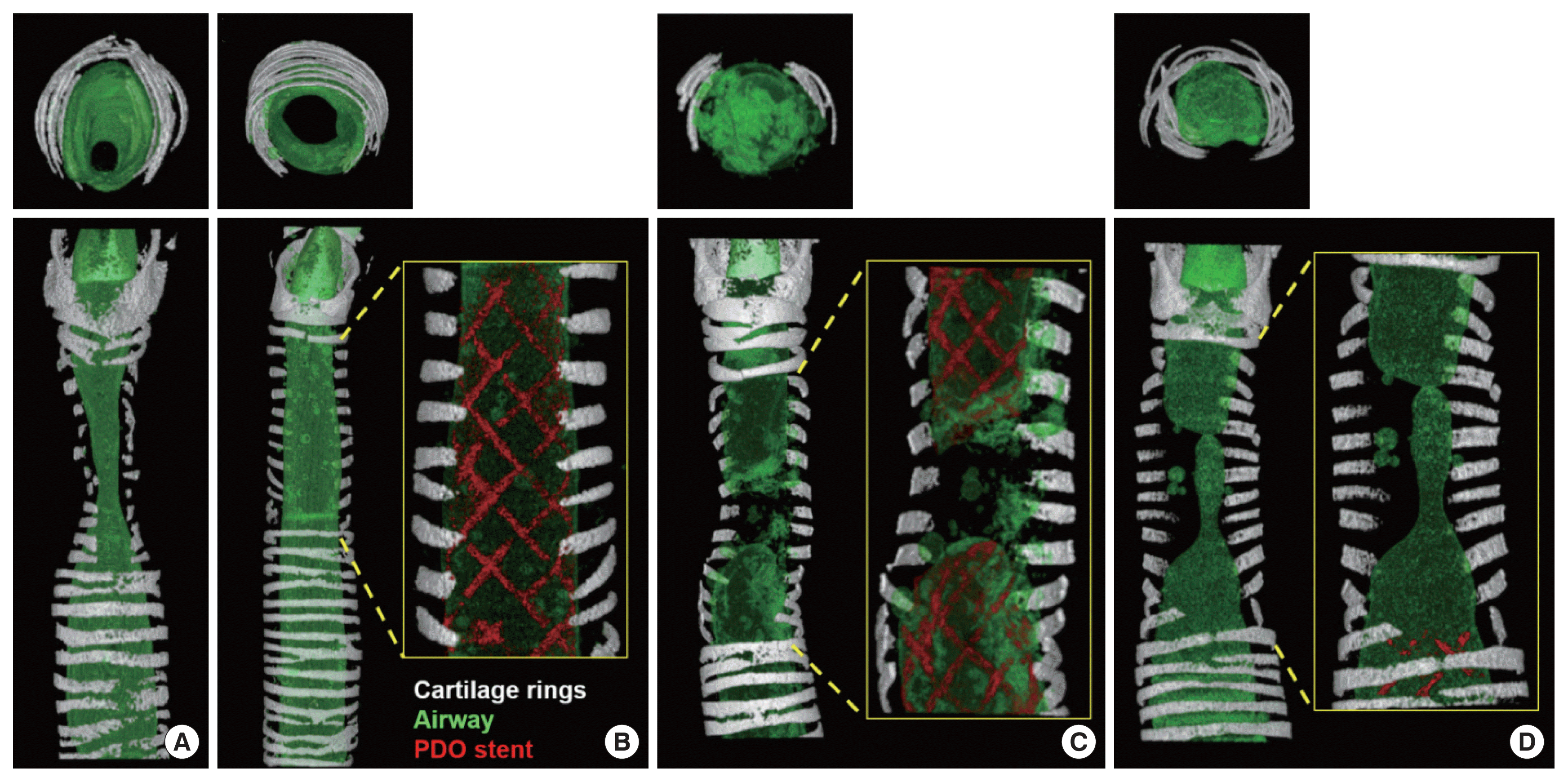
11. Vondrys D, Elliott MJ, McLaren CA, Noctor C, Roebuck DJ. First experience with biodegradable airway stents in children. Ann Thorac Surg. 2011; Nov. 92(5):1870–4.

12. Sztano B, Kiss G, Marai K, Racz G, Szegesdi I, Racz K, et al. Biodegradable airway stents infants: potential life-threatening pitfalls. Int J Pediatr Otorhinolaryngol. 2016; Dec. 91:86–89.
13. Novotny L, Crha M, Rauser P, Hep A, Misik J, Necas A, et al. Novel biodegradable polydioxanone stents in a rabbit airway model. J Thorac Cardiovasc Surg. 2012; Feb. 143(2):437–44.

14. Kawahara I, Ono S, Maeda K. Biodegradable polydioxanone stent as a new treatment strategy for tracheal stenosis in a rabbit model. J Pediatr Surg. 2016; Dec. 51(12):1967–71.

15. Zamiri P, Kuang Y, Sharma U, Ng TF, Busold RH, Rago AP, et al. The biocompatibility of rapidly degrading polymeric stents in porcine carotid arteries. Biomaterials. 2010; Nov. 31(31):7847–55.

16. Zhou X, Pan Y, Liu R, Luo X, Zeng X, Zhi D, et al. Biocompatibility and biodegradation properties of polycaprolactone/polydioxanone composite scaffolds prepared by blend or co-electrospinning. J Bioact Compat Polym. 2019; 34(2):115–30.

17. Yanhui L, Ruitao J, Mian W, Shaoju F, Peihua Z. Degradation and biocompatibility behaviors of fully covered biodegradable polydioxanone biliary stent for human body. Ind Textila. 2019; 70(5):393–7.
18. Hakimi O, Murphy R, Stachewicz U, Hislop S, Carr AJ. An electrospun polydioxanone patch for the localisation of biological therapies during tendon repair. Eur Cell Mater. 2012; Oct. 24:344–57.

19. Li G, Li Y, Lan P, Li J, Zhao Z, He X, et al. Biodegradable weft-knitted intestinal stents: fabrication and physical changes investigation in vitro degradation. J Biomed Mater Res A. 2014; Apr. 102(4):982–90.

20. Stehlik L, Hytych V, Letackova J, Kubena P, Vasakova M. Biodegradable polydioxanone stents in the treatment of adult patients with tracheal narrowing. BMC Pulm Med. 2015; Dec. 15:164.

21. Vondrys D, Anton-Pacheco Sanchez J. Letter to the editor regarding “Biodegradable airway stents in infants: potential life-threatening pitfalls”. Int J Pediatr Otorhinolaryngol. 2017; Jul. 98:174.
22. Miller FR, Guay ME, Bauer T, Tucker HM. Long-term flap tracheostomy in a pediatric animal model. Arch Otolaryngol Head Neck Surg. 1995; Jul. 121(7):743–8.

23. Gorostidi F, Courbon C, Burki M, Reinhard A, Sandu K. Extraluminal biodegradable splint to treat upper airway anterior malacia: a preclinical proof of principle. Laryngoscope. 2018; Feb. 128(2):E53–8.

24. Filler RM, Messineo A, Vinograd I. Severe tracheomalacia associated with esophageal atresia: results of surgical treatment. J Pediatr Surg. 1992; Aug. 27(8):1136–40.

25. Hysinger EB, Panitch HB. Paediatric tracheomalacia. Paediatr Respir Rev. 2016; Jan. 17:9–15.

26. Saito Y, Minami K, Kobayashi M, Nakao Y, Omiya H, Imamura H, et al. New tubular bioabsorbable knitted airway stent: biocompatibility and mechanical strength. J Thorac Cardiovasc Surg. 2002; Jan. 123(1):161–7.

27. Liu KS, Liu YH, Peng YJ, Liu SJ. Experimental absorbable stent permits airway remodeling. J Thorac Cardiovasc Surg. 2011; Feb. 141(2):463–8.

28. Lochbihler H, Hoelzl J, Dietz HG. Tissue compatibility and biodegradation of new absorbable stents for tracheal stabilization: an experimental study. J Pediatr Surg. 1997; May. 32(5):717–20.

29. Korpela A, Aarnio P, Sariola H, Tormala P, Harjula A. Comparison of tissue reactions in the tracheal mucosa surrounding a bioabsorbable and silicone airway stents. Ann Thorac Surg. 1998; Nov. 66(5):1772–6.

30. Korpela A, Aarnio P, Sariola H, Tormala P, Harjula A. Bioabsorbable self-reinforced poly-L-lactide, metallic, and silicone stents in the management of experimental tracheal stenosis. Chest. 1999; Feb. 115(2):490–5.

31. Robey TC, Valimaa T, Murphy HS, Tormala P, Mooney DJ, Weatherly RA. Use of internal bioabsorbable PLGA “finger-type” stents in a rabbit tracheal reconstruction model. Arch Otolaryngol Head Neck Surg. 2000; Aug. 126(8):985–91.

32. Wang CE, Zhang PH. In vitro degradation behaviours of PDO monofilament and its intravascular stents with braided structure. Autex Res J. 2016; 16(2):80–9.

33. Sabino MA, Gonzalez S, Marquez L, Feijoo JL. Study of the hydrolytic degradation of polydioxanone PPDX. Polym Degrad Stab. 2000; 69(2):209–16.

34. Gefen A, Weihs D. Cytoskeleton and plasma-membrane damage resulting from exposure to sustained deformations: a review of the mechanobiology of chronic wounds. Med Eng Phys. 2016; Sep. 38(9):828–33.

35. Hill GR. Host γδ T cells: an innate bridge to the epithelial targets of GVHD? Blood. 2005; Jul. 106(2):393–4.