Abstract
Background
Pregnancy-related infections are the third most common cause of maternal death worldwide. The aim of this report is to present a case of pregnancy-related infection, which progressed into refractory septic shock accompanied by purpura fulminans and multiple organ failure.
Case
A 23-year-old woman in the postpartum period developed fulminant, refractory septic shock complicated by purpura fulminans and multiple organ failure syndrome (acute respiratory distress syndrome, acute kidney injury, and encephalopathy). Management included antibacterial therapy, fluid and transfusion therapy, nutritional support, protective mechanical ventilation, hydrocortisone, a large dose of ascorbic acid, and thiamine. There were no neurological consequences and all organ functions returned to normal, although the predicted hospital mortality based on the Sequential Organ Failure Assessment (SOFA) score was more than 90%.
Pregnancy-related infection is the third most common cause of maternal death worldwide [1]. Sepsis represents one of the common causes of pregnancy-related mortality, the most frequent being hypertension, abortion, and hemorrhagic complications [2]. The most common conditions and procedures leading to severe infection and sepsis in obstetrics include chorioamnionitis, septic thrombophlebitis, septic abortion, postpartum endometritis, puerperal sepsis, infection after cesarean section, episiotomy, wound infection, necrotizing fasciitis, pelvic abscess as well as hospital acquired infections such as ventilator-associated pneumonia, urinary tract infection, and central line-associated infection [3]. Refractory septic shock is defined as “the presence of hypotension with end-organ failure, requiring high-dose vasopressor support, often greater than 0.5 μg/kg/min of norepinephrine or equivalent” [4]. Despite recent advances, it is associated with a mortality rate of 15–50%; furthermore, if the required dose of norepinephrine exceeds 1.0 μg/kg/min, the mortality can be as high as 80–90% [5]. The key component of refractory shock is severe tissue hypoperfusion, and critical cellular and metabolic failure.
Lipopolysaccharide is a component of the outer membrane of gram-negative bacteria and is one of the key causative factors of septic shock in ICU patients [6]. The intravasation of gram-negative bacteria can trigger a cascade of systemic inflammatory reactions that frequently result in lethal outcomes [7]. Purpura fulminans is a serious condition, which usually occurs secondary to sepsis; it can be associated with disseminated intravascular coagulation (DIC) and is characterized by dermal vascular thrombosis and hemorrhagic infarction of the skin [8]. Sepsis-induced purpura fulminans involves an imbalance of anticoagulant and procoagulant activities of the endothelial cells [9]. This imbalance is induced by the endotoxins or exotoxins in gram-negative or gram-positive sepsis respectively, mediated by cytokines, resulting in the consumption of proteins C and S, and antithrombin III [9]. There are three main etiological subtypes of purpura fulminans: 1) idiopathic purpura fulminans–occurs in patients without known or acute infections or abnormalities of the protein C pathway, 2) acute infectious purpura fulminans–occurs mainly in patients with acute severe gram-negative bacterial infections, 3) occurs in patients with preexistent inherited or acquired abnormality of protein C or S anticoagulant pathway [9]. In this case report, we present a case of pregnancy-related infection, which progressed into refractory septic shock accompanied by purpura fulminans and multiple organ failure.
A 23-year-old postpartum woman with septic shock was transferred to the intensive care unit of the University Medical Center, from a regional rural hospital. During pregnancy, she was diagnosed with severe iron deficiency anemia (hemoglobin concentration 8.6 g/dl), gestational hypertension, meconium-stained amniotic fluid, and prolonged rupture of the membranes. There was no evidence of infection during the period of pregnancy and no Streptococcus spp. was detected in the vaginal smear. The course of pregnancy was complicated by chorioamnionitis, and urgent cesarean section was performed several days before transfer to our department. There was no evidence of shock/end-organ dysfunction during labor. About 24 hours following cesarean section, the patient developed clinical manifestations of purulent postpartum endometritis with fever of 39°C, chills, purulent uterine discharge, and fundal tenderness. An arterial line was placed following the diagnosis of endometritis. The sample for blood culture was obtained before initiation of empiric antibacterial therapy (vancomycin and gentamicin). Several hours later, she developed sepsis that abruptly progressed to septic shock (arterial pressure decreased to 60/40 mmHg) and organ failure with a sequential organ failure assessment (SOFA) score of 16–20 (10–12 points on the Glasgow Coma Scale, acute respiratory distress syndrome [ARDS] with PaO2/FiO2 180 mmHg, norepinephrine > 0.3 μg/kg/min [weight 60 kg], bilirubin 23 mmol/L, platelets 35 × 103/μl, and urine output 300 ml/day). Laboratory tests showed multiple abnormalities including hemoglobin concentration of 7.7 g/dl, white blood cells 25×109/L, prothrombin time 21 s, fibrinogen concentration 89 mg/dl, antithrombin III 53%, D-Dimer 2.4 µg/ml (normal range < 0.5), C-reactive protein 309.5 mg/L, procalcitonin 45 ng/ml, lactate 7.5 mmol/L, and brain natriuretic peptide (BNP) 4,000 ng/ml (Table 1). Protein C and protein S tests were not available due to technical limitations. A bolus of normal saline (30 ml/kg) was administered and immediate norepinephrine infusion (0.3 μg/kg/min) was initiated. Due to the patient’s unstable condition, she was intubated and respiratory support in assist-control mode was initiated. Within a short span of time, the dose of norepinephrine was increased to 3.0 μg/kg/min to maintain the mean arterial pressure in the range of 60–65 mmHg. Two hours after the onset of shock, the color of her fingers and toes as well as abdominal wall appeared mottled. Several hours later, the echocardiogram demonstrated left ventricular systolic dysfunction with an ejection fraction of 32%, and dobutamine infusion (20 μg/kg/min) was initiated to improve cardiac contractility. Attempts to reduce the doses of catecholamines were unsuccessful due to inadequate hemodynamic response to fluid therapy. After initial stabilization of the puerperal condition, hysterectomy was performed. Two days later, the patient was transferred to our hospital by sanitary aviation.
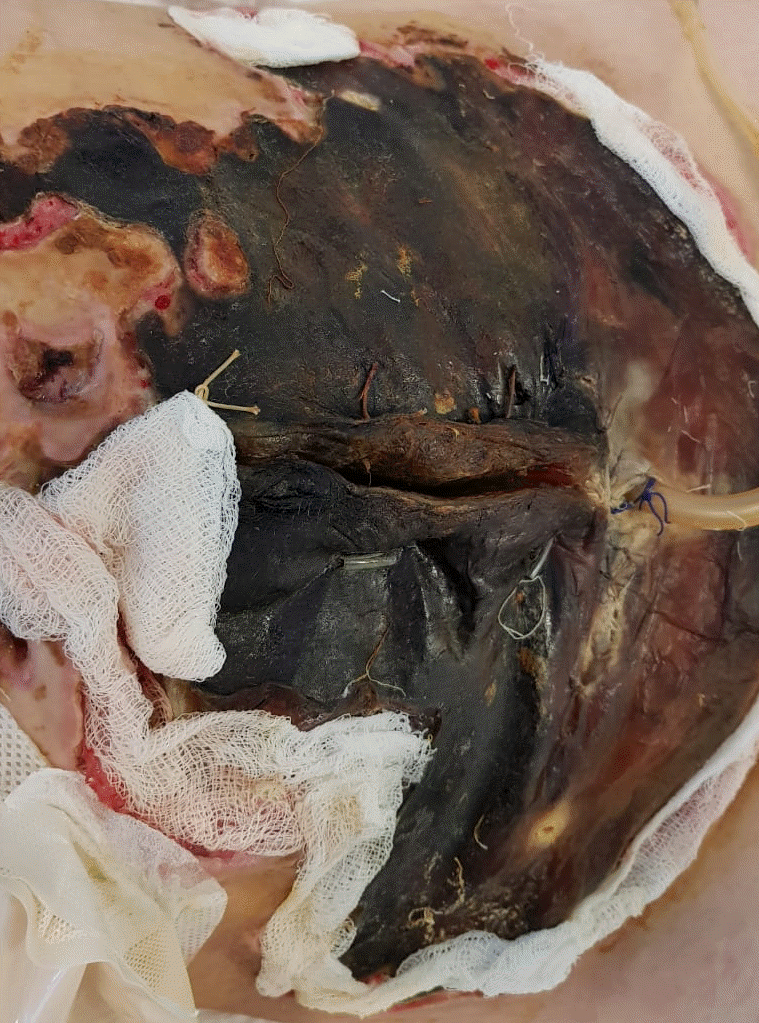
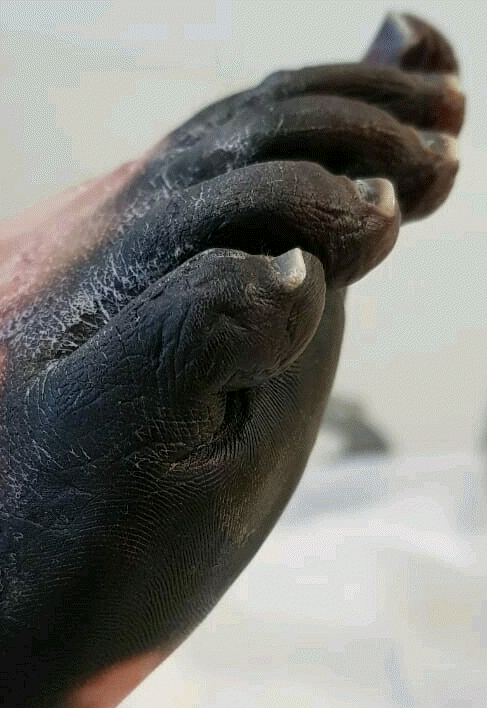
Physical examination on admission to our department revealed massive aseptic necrosis of all fingers (I–V), toes (I–V), metatarsal region, and anterior abdominal wall (Figs. 1 and 2). The patient also had had sepsis/septic shock-induced multiple system failure including ARDS, encephalopathy, systolic heart failure, and acute kidney injury. Invasive blood pressure was 100/70 mmHg, heart rate was 150/min, arterial oxygen saturation was 85–92%, and body temperature was 38.4°C. The patient was sedated with dexmedetomidine (0.4 μg/kg/h) and mechanically ventilated (assist/control ventilation with positive end expiratory pressure of 14 cmH2O and FiO2 0.7). Auscultation revealed crackles over the right middle and lower zones of the lungs. Hemodynamic support required intravenous norepinephrine 2 μg/kg/min and dobutamine 20 μg/kg/min.
Investigations revealed the following abnormalities: Hb 9 g/dl, WBC 22 × 109/L, C-reactive protein 200.5 mg/L, lactate 4 mmol/L, procalcitonin 32 ng/ml, сreatine-kinase MB 15.1 IU/L, BNP 5,000 ng/ml, platelets 90 × 109/L, fibrinogen 114 mg/dl, activated partial thromboplastin time 128 s, –international normalized ratio 1.27, prothrombin time 16.9 s (Table 1). Transthoracic echocardiography demonstrated reduced (but improved compared to the previous values) ejection fraction of 38%. Chest radiography showed bilateral infiltrations (mild acute respiratory distress syndrome with PaO2/FiO2 250 mmHg). Blood culture showed multiple drug-resistant Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii (all sensitive to meropenem). No microbial pathogens were identified in the necrotic tissues. Patient management plan included antibacterial therapy, goal-directed fluid therapy, nutritional support, protective mechanical ventilation, hydrocortisone, fresh frozen plasma, and high dose of ascorbic acid and thiamine. The skin wounds were managed with dressing and moist healing products. Three weeks after admission to our intensive care unit the patient was weaned off mechanical ventilation as well as from vasopressor therapy. There were no neurological consequences and all end-organ functions returned to normal (although the SOFA score-predicted hospital mortality was more than 90%); however, the necrotic area was quite large. The duration of vasopressor support totaled 8 days. After stabilization of the general condition, the necrotic masses were debrided and autodermoplasty was performed signed informed consent for publication was obtained.
In our patient, refractory septic shock was complicated by purpura fulminans-a relatively infrequent symptom of DIC [10], which resulted in progressive tissue necrosis.
The presentation of such a massive lesion can be explained by the critical reduction of tissue perfusion due to purpura fulminans, probable heterogeneity in the distribution of blood flow, endothelial injury, and impaired oxygen utilization. Success in septic shock management depends on early recognition and resuscitation. The outcomes of treatment can be improved and complications can be minimized by a balanced combination of fluid and vasopressor/inotropic therapy; both should be initiated as soon as possible to improve microcirculation, blood viscosity, and to normalize coagulation to prevent severe ischemia-hypoxic tissue injury. Vasopressor therapy is one of the key components of septic shock management, especially if early fluid therapy cannot achieve hemodynamic stabilization. However, high-dose vasoconstrictor therapy in a hypovolemic patient might result in exacerbation of tissue ischemia and development of irreversible ischemic injuries such as digital necrosis as well as necrosis of the internal organs. Advanced hemodynamic monitoring might be useful for the customization of fluid and vasopressor/inotropic therapy.
This condition was complicated by the failure of cardiovascular, pulmonary, cerebral, renal, and intestinal systems, and massive necrosis of the skin and subcutaneous tissue. Another interesting finding in our patient was that she developed sepsis-induced cardiac dysfunction several hours after the onset of sepsis. BNP was significantly elevated to 5,000 ng/ml and the ejection fraction was severely reduced to 32%, although the patient had no history of cardiac problems. It has been reported that cardiac dysfunction in patients with septic shock was associated with a significant increase in the rate of mortality up to 70–90%, compared to 20% in those without cardiac dysfunction [11].
The clinical manifestations of sepsis-induced purpura fulminans commonly include purpuric rash as well as symptoms and signs of sepsis, which is usually preceded by fever or chills, sore throat, and malaise. It has been reported that purpura fulminans was associated in about 67% of patients with septic shock and in 78% of patients with DIC [12]. The differential diagnoses include thrombotic thrombocytopenic purpura, postinfectious thrombocytopenic purpura, and Henoch-Schonlein purpura [12]. Purpura fulminans can be associated with a mortality of up to 50%. The standard management of purpura fulminans includes aggressive fluid resuscitation, inotropic therapy, respiratory support, coagulation control and management (fresh frozen plasma, anticoagulants), treatment of underlying infection, renal replacement therapy, and management of complications [13]. In addition, we believe that our patient might have benefited by early initiation of blood purification therapy with cytosorbent or its analogues. However, it is uncertain whether this would have prevented the development of purpura fulminans and massive necrosis, since the onset of septic shock was abrupt and blood purification therapy would require time before visible effect. In our opinion, early removal of the endotoxins and inflammatory cytokines could probably attenuate multiple organ dysfunction and reduce the extent of organ failure.
In conclusion, septic shock is a significant, yet not completely understood life-threatening condition, which can be associated with purpura fulminans, multiple organ dysfunction, disseminated intravascular coagulation, and massive tissue necrosis.
References
1. Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014; 2:e323–33.

2. Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006; 367:1066–74.

3. Cordioli RL, Cordioli E, Negrini R, Silva E. Sepsis and pregnancy: do we know how to treat this situation? Rev Bras Ter Intensiva. 2013; 25:334–44.

4. Bassi E, Park M, Azevedo LC. Therapeutic strategies for high-dose vasopressor-dependent shock. Crit Care Res Pract. 2013; 2013:654708.

5. Brown SM, Lanspa MJ, Jones JP, Kuttler KG, Li Y, Carlson R, et al. Survival after shock requiring high-dose vasopressor therapy. Chest. 2013; 143:664–71.

6. Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994; 8:217–25.

7. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003; 348:1546–54.

8. Marlar RA, Neumann A. Neonatal purpura fulminans due to homozygous protein C or protein S deficiencies. Semin Thromb Hemost. 1990; 16:299–309.

9. Adcock DM, Brozna J, Marlar RA. Proposed classification and pathologic mechanisms of purpura fulminans and skin necrosis. Semin Thromb Hemost. 1990; 16:333–40.

10. Cordioli RL, Cordioli E, Negrini R, Silva E. Sepsis and pregnancy: do we know how to treat this situation? Rev Bras Ter Intensiva. 2013; 25:334–44.

11. Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, et al. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990; 113:227–42.
Table 1.
Clinical and Laboratory Characteristics of Multiple Organ Dysfunction in Our Patient




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download