Abstract
Purpose
We performed this study to determine whether the degree of neutropenia after the first chemotherapy cycle can be used as a surrogate marker of individual susceptibility to chemotherapeutic agents affecting treatment outcome in patients with neuroblastoma.
Materials and Methods
The study included 313 patients who received the first cycle chemotherapy with a CEDC (cisplatin+etoposide+doxorubicin+cyclophosphamide) regimen and had absolute neutrophil count (ANC) data available. The cumulative incidences of progression and treatment-related mortality (TRM) were estimated. To identify genetic variations associated with the ANC, a genome-wide association study (GWAS) was performed.
Results
An ANC of 32.5/μL was determined as the cutoff point to categorize patients into the good and poor prognosis subgroups in terms of progression. Patients with a high nadir ANC had a higher cumulative incidence of progression than those with a low nadir ANC (p < 0.001). In multivariate analysis, high nadir ANC, age, bone marrow involvement, and unfavorable histology were poor prognostic factors. With regard to the TRM, patients with a low nadir ANC (ANC < 51.0/μL) had a higher cumulative incidence of TRM than those with a high nadir ANC (p=0.010). In GWAS, single-nucleotide polymorphisms of LPHN2 and CRHR1 were significantly associated with the nadir ANC.
Neuroblastoma (NB) is the most common extracranial solid tumor in children, accounting for 6%–10% of all childhood cancers [1]. It has a heterogeneous course depending on its clinical and biological features [2,3]. Patients with NB are stratified into risk groups according to various clinical and biological risk factors, including age at diagnosis, stage, pathology, and MYCN amplification. All patients in a particular risk group receive the same treatment. However, treatment outcomes, including toxicity and survival during or after treatment, vary among patients, possibly because of differences in the somatic characteristics of the tumor or because individuals have varying susceptibility to chemotherapeutic agents.
Individual variation in susceptibility to chemotherapeutic agents may occur because of differences in germline genomics, including single-nucleotide polymorphisms (SNPs). Many pharmacogenomic studies have shown that SNPs are useful predictive biomarkers for drug-induced adverse events and drug response [4,5]. Moreover, several studies have shown that polymorphisms of candidate genes involved in drug metabolism are responsible for variable drug toxicity among patients with leukemia [6,7]. However, limited studies have investigated the effect of individual drug susceptibility on treatment outcome in pediatric solid tumors.
In the present study, we hypothesized that the degree of neutropenia after the first chemotherapy cycle could be used as a surrogate marker of individual susceptibility to chemotherapeutic agents and that it affects treatment outcome. We analyzed whether the absolute neutrophil count (ANC) at the nadir after the first chemotherapy cycle is associated with treatment outcome in patients with NB. In addition, we determined whether germline genetic polymorphisms can affect the degree of neutropenia using a genome-wide association study (GWAS).
Patients who were diagnosed with NB and received chemotherapy between March 2000 and December 2018 were screened. Patients who underwent the first cycle chemotherapy with a cisplatin+etoposide+doxorubicin+cyclophosphamide (CEDC) regimen and had ANC data available from the first cycle chemotherapy were included in this study. In total, 313 patients were enrolled in the present study. The patients’ medical records were reviewed for detailed clinical and biological data, including clinical features at diagnosis, tumor biology (including MYCN amplification), tumor histology according to the International Neuroblastoma Pathology Classification (INPC), treatment, survival, and treatment toxicity. The following equation was calculated to measure tumor volume: (π/6)×depth×width×height [8]. In the GWAS, the analytic cohort comprised 269 patients whose peripheral blood samples were cryopreserved at the Samsung Medical Center Biobank.
Patients were classified into three risk groups according to age at diagnosis, stage according to the International Neuroblastoma Staging System (INSS), and MYCN amplification. In brief, stage 1 or 2 tumors without MYCN amplification were classified as low-risk tumors. Stage 4 tumors in patients aged > 12 months (before 2015) or > 18 months (from 2015) and MYCN-amplified tumors were classified as high-risk tumors. All other tumors were classified as intermediate-risk tumors. For chemotherapy, CEDC and ifosfamide+carboplatin+etoposide (ICE) regimens were used alternatively. Detailed information on the chemotherapy regimen has been described in a previous report [9]. In general, low-risk patients with stage 2 tumors received six cycles of chemotherapy; intermediate-risk patients received nine cycles of chemotherapy plus differentiation therapy (13-cis-retinoic acid) with or without local radiotherapy to the primary site; and high-risk patients received nine cycles of induction chemotherapy, followed by tandem high-dose chemotherapy (HDCT), local radiotherapy, and differentiation therapy with or without immunotherapy with interleukin 2 [9,10]. Therefore, all patients received the same chemotherapy regimen for the first six cycles. A smaller chemotherapy dose was used in patients aged < 24 months as follows: a body weight-based dose in patients aged < 6 months, a 70% dose based on body surface area in patients aged 6–11 months, an 80% dose in patients aged 12–17 months, and a 90% dose in patients aged 18–23 months. From the second cycle, the chemotherapy dose was reduced by 20–30% if the patients suffered from severe sepsis during the previous cycle or if the hematologic recovery took more than 42 days. Granulocyte colony-stimulating factor was used from the day when the ANC < 500/μL until the time when ANC > 1,000/μL.
Genomic DNA was extracted from the patients’ peripheral blood lymphocytes using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI) according to the manufacturer’s protocol. Approximately 250 ng of genomic DNA was used to genotype each sample using Illumina’s Global Screening Array BeadChip (Illumina, San Diego, CA). The samples were then processed according to the Illumina Infinium assay manual. The quality of the sample was checked based on the sample call rate (> 95%). The clustering quality of each marker was measured based on the GenTrain score. Markers with a score of > 0.7 were used in the study. We performed imputation using the Michigan Imputation Server (https://imputationserver.sph.umich.edu). Markers with low imputation quality (defined as a call rate < 98%), minor allele frequency (MAF) < 1%, a p-value of Hardy-Weinberg equilibrium < 1e–5, duplicated markers, and ambiguous strand markers were excluded from the association analysis. Low-quality samples with a call rate < 95% were used for quality control.
Maximally selected log-rank statistics were tested to obtain the optimal ANC cutoff values that categorized patients into the good and poor prognosis subgroups in terms of progression and treatment-related mortality (TRM) [11]. Cumulative incidences of progression and TRM were estimated using competing risk methods by considering progression and TRM as competing risks [12]. The cumulative incidences of progression and TRM were compared using the Gray’s test. The Fine and Gray competing risk regression model was used to evaluate the effect of the ANC and clinical covariates on the cumulative incidences of progression and TRM. In this analysis, progression and TRM were defined as events, and event-free survival (EFS) was calculated from the date of diagnosis until the date of progression or TRM, whichever occurred first. Overall survival (OS) was calculated from the date of diagnosis until death from any cause. The EFS and OS rates were estimated using the Kaplan-Meier method, and differences in survival curves were compared using the log-rank test. Clinical characteristics were compared between the two groups using the Pearson chi-square test or Fisher exact test for categorical variables and the t test or Kruskal-Wallis rank-sum test for continuous variables. All statistical analyses were performed using R ver. 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria), and p < 0.05 were considered statistically significant.
To investigate significant markers, numeric regression analyses were performed on the ANC with genotypes using HelixTree software (Golden Helix Inc., Bozeman, MT). To assess multiple corrections, the false discovery rate was used. The DAVID functional annotation tool (https://david.ncifcrf.gov/) was used for the analysis. Gene pathway analysis was performed to detect SNPs significantly associated with the ANC after chemotherapy. To visualize significant regional markers, Locus zoom plotting was performed using LocusZoom [13].
Altogether, 313 patients were enrolled in the current study. The median age at diagnosis was 2.4 years (range, 0.0 to 19.2 years). One hundred ninety-one patients (61.0%) had metastatic disease and 110 (35.1%) patients had bone marrow metastasis. Tumor histology according to the INPC was favorable in 137 patients (43.8%), unfavorable in 154 patients (49.2%), and unknown in 22 patients (7.0%). Cytogenetic analysis was only performed in some patients; 68 of 310 patients (21.9%) had MYCN amplification, 17 of 124 patients (13.7%) had 1p deletion, 35 of 125 patients (28.0%) had 11q deletion, and 33 of 123 patients (26.8%) had 17q gain. A total of 173 patients (55.1%) had high-risk tumors.
During the study period, 59 patients showed progression and 25 experienced TRM; the 5-year EFS and OS rates were 73.1%±2.6% and 80.7%±2.3%, respectively. In high-risk patients, progression and TRM occurred in 51 and 21 patients, respectively, and the 5-year EFS and OS rates were 58.6%±3.9% and 69.0%±3.7%, respectively.
First, we analyzed whether the degree of neutropenia after the first cycle of chemotherapy was associated with the cumulative incidence of progression. Maximally selected log-rank statistics determined that an ANC of 32.5/μL was an optimal cutoff point to categorize patients into the good and poor prognosis subgroups in terms of progression. The patient characteristics in the nadir ANC subgroups are presented in Table 1. The frequency of bone marrow involvement did not differ between the two groups. Patients with a high nadir ANC had a higher cumulative incidence of progression than those with a low nadir ANC (p < 0.001). The 5-year cumulative incidences of progression were 12.6%±0.1% and 30.3%±0.2% in the low and high nadir ANC groups, respectively. However, the EFS and OS did not differ between the two groups (p=0.084 and p=0.428, respectively) because TRM showed opposite outcome (high TRM rate in those with a low nadir ANC) (Fig. 1A). Multivariate analysis using the Fine and Gray competing risk regression showed that high nadir ANC, age, bone marrow involvement, and unfavorable tumor histology were poor prognostic factors for the cumulative incidence of progression (Table 2).
Because age was significantly different between the two groups and patients aged < 2 years received chemotherapy at a reduced dose, patients aged > 2 years were included in subgroup analysis. In subgroup analysis, patients with a low nadir ANC had a better outcome in terms of cumulative incidence of progression than those with a high nadir ANC (p=0.004), but the EFS and OS did not differ among the groups (p=0.051 and p=0.304, respectively) (Fig. 1B). The age distribution was different between the two groups (median, 3.3 years in the low nadir ANC group vs. 4.2 years in the high nadir ANC group; p=0.022). Since the ANC might have served as a confounding factor in the analysis of age and outcome, multivariate analysis was conducted using both age and the nadir ANC as covariates. Only high nadir ANC had a significant effect on the cumulative incidence of progression (Table 2).
In patients with high-risk NB, the difference in the cumulative incidence of progression and EFS became more prominent (Fig. 1C), and multivariate analysis showed that only high nadir ANC was significantly associated with the cumulative incidence of progression (Table 2). In the analysis of only non–high-risk patients, there were no significant differences in the cumulative incidence of progression or survival among the nadir ANC groups.
The primary tumor volume was measured at diagnosis and at the first response evaluation after three cycles of induction chemotherapy. The percentage tumor volume at the first response evaluation compared with the initial tumor volume was calculated. Tumor volume was only evaluated in 164 patients who had undergone three-dimensional computed tomography or magnetic resonance imaging at diagnosis and had not undergone front-line surgery at diagnosis. Patients with a low nadir ANC showed greater tumor volume reduction than those with a high nadir ANC (median residual volume %: 17.5% vs. 47.8%, respectively; p=0.004) (Fig. 2A). This difference was more prominent in high-risk patients (median residual volume %, 16.3% vs. 49.7%; p=0.001) (Fig. 2B).
Subgroup analysis only including undifferentiated or poorly differentiated NB was performed because histological tumor differentiation, which can affect the tumor response, was different between the two groups (Table 1). When the analysis was confined to patients with undifferentiated or poorly differentiated NB, those with a low nadir ANC still showed greater tumor volume reduction than those with a high nadir ANC (median, 14.4% vs. 27.3%; p=0.003) (Fig. 2C).
TRM occurred in 25 patients—three patients died during induction chemotherapy, 11 patients showed acute TRM after HDCT, nine patients had late TRM after HDCT, and two patients died due to secondary malignancy. Maximally selected log-rank statistics showed that an ANC of 51.0/μL was the optimal cutoff point to categorize patients based on TRM. Patients with a low nadir ANC (≤ 51.0/μL) showed a higher cumulative incidence of TRM than those with a high nadir ANC (p=0.010). The 5-year cumulative incidences of TRM were 9.43%±0.04% and 1.25%±0.02% in the low and high nadir ANC groups, respectively (Fig. 3A). The EFS and OS did not differ between the two groups. In the analysis of high-risk patients, the cumulative incidence of TRM was higher in patients with a high nadir ANC than in those with a low nadir ANC (Fig. 3B).
A quantile–quantile plot of the association test using the ANC showed a significant deviation of measures at the tail (S1 Fig.), indicating potentially true associations between SNPs and the ANC. The GWAS of common SNPs (MAF > 0.05), which were associated with the ANC, are represented in a Manhattan plot (Fig. 4). The top 30 SNP loci associated with the ANC are listed in S2 Table. A SNP at rs4285743 in the intron of LPHN2 showed the most strong association with the ANC (p=2.9e–09 and Pcorr=0.002); a SNP at rs143699161 in the intron of CRHR1 was also significantly associated with the ANC (p=6.1e–09 and Pcorr=0.004). To examine the biological function of these SNPs, we performed gene ontology (GO) analysis using DAVID, and the results were presented in S3 Table. Eleven GO terms were observed to have significantly corrected p < 0.05. A number of GO terms related to neural development and synaptic signaling were isolated.
In the present study, we investigated whether the degree of neutropenia after the first chemotherapy cycle could be used as a surrogate marker for determining an individual’s susceptibility to chemotherapeutic agents and its effect on treatment outcome in patients with NB. The final outcomes such as EFS and OS did not differ according to the nadir ANC group in all patients. However, this was because the ANC nadir group was inversely associated with the cumulative incidence of progression and TRM (even though the ANC cutoff points were different between progression and TRM). In other words, the patients in the high nadir ANC group showed a higher cumulative incidence of progression, but the cumulative incidence of TRM was higher in patients in the low nadir ANC group. These findings suggest that patients who are more susceptible to chemotherapeutic agents could have profound neutropenia after the first cycle of chemotherapy and have better outcomes in terms of progression. However, these patients are more vulnerable to toxicities. Collectively, our findings suggest that the degree of neutropenia could be used as a clinical marker to predict an individual’s susceptibility to chemotherapeutic agents, and tailoring of treatment based on the degree of neutropenia needs to be considered.
Several studies have shown an association between chemotherapy-induced neutropenia and survival outcome in adult patients with cancer [14–17]. Specifically, the early onset of neutropenia during or after chemotherapy was associated with better OS. A study including children with acute myeloid leukemia (AML) showed that longer neutropenia duration was associated with a reduced risk of relapse in children with favorable and standard-risk AML [18]. These studies suggest that patients have different abilities to metabolize chemotherapeutic drugs and that poor metabolizers have a greater drug exposure, resulting in significant bone marrow suppression and fewer relapses. The present study showed similar results; patients who developed profound neutropenia showed lower progression rates and a greater tumor volume response, possibly because tumor cells share a patient’s genetic characteristics.
TRM is an extreme form of treatment-related toxicity and is particularly common in children receiving intensive chemotherapy, which can result in infection, bleeding, or organ dysfunction [19]. In our study population, most TRM occurred in patients who underwent tandem HDCT. TRM occurred less frequently in patients with a high nadir ANC (> 51.0/μL) than in those with a low nadir ANC (ANC ≤ 51.0/μL), suggesting that patients who could metabolize drugs better were more tolerant to chemotherapeutic agents and, therefore, had milder bone marrow suppression and decreased risk of TRM. However, the EFS or OS outcomes were not different because the degree of neutropenia had the opposite effect on progression—patients with milder neutropenia showed a higher progression rate.
The effects of the degree of neutropenia on treatment outcome were more prominent when the analysis was confined to high-risk patients who received very intensive treatment, including tandem HDCT. Conversely, the effects of the degree of neutropenia were not significant in low- or intermediate-risk patients, suggesting that treatment outcome is not significantly affected by the degree of neutropenia when treatment is less intensive, such as that in the low- and intermediate-risk patients in the present cohort. Therefore, in high-risk patients who receive intensive treatment, tailoring of treatment based on the degree of neutropenia needs to be considered. A prospective study is needed to investigate whether personalized treatment according to the degree of neutropenia can improve the final survival rate by reducing the cumulative incidence of progression and TRM in patients with high-risk NB.
We performed the GWAS to identify genetic factors responsible for the variable degree of neutropenia after the same chemotherapy; SNPs of LPHN2 and CRHR1 were significantly associated with the degree of neutropenia. LPHN2 is predicted to play a role in cell signaling linked to adhesion [20]. Intronic SNPs of LPHN2 have been reported to be associated with decreased paclitaxel sensitivity in cancer cell lines, and the authors proposed that microtubule interactions of LPHN2 could play an important role in paclitaxel response [21]. In another study involving advanced breast cancer, a SNP of LPHN2 was significantly related to poor tumor response [22]. CRHR1 encodes a receptor that binds neuropeptides of the corticotropin-releasing hormone family. No previous studies have reported any association of this gene with chemotherapy response or toxicity. Further studies are needed to confirm the clinical significance of these polymorphisms in the treatment outcomes of NB.
The number of patients in our cohort was lower than that in previous adult studies because NB is rare. Furthermore, patients in our cohort received treatment according to our own protocols, including intensive tandem HDCT in high-risk patients, which was uncommon globally during the study period. Hence, we could not validate the findings of the present study in another cohort. Therefore, our findings need to be confirmed in a larger cohort of children and adolescents with NB or other solid tumors.
In conclusion, the degree of neutropenia after the first chemotherapy cycle could be used as a surrogate marker to predict an individual’s susceptibility to chemotherapeutic agents, and thus, it can predict treatment outcomes in patients with NB. Further confirmatory studies on genetic markers are needed to explain the results of the present study. In addition, tailoring of treatment based on the degree of neutropenia needs to be considered, especially in high-risk patients.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
Written informed consent was obtained from the parents or guardians of each patient. This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB No. SMC 2015-06-068 and SMC 2020-12-164).
Author Contributions
Conceived and designed the analysis: Lee JW, Sung KW.
Collected the data: Lee JW, Cho HW, Ju HY, Yoo KH, Koo HH.
Contributed data or analysis tools: Bae JS, Kim JH, Woo SY, Kim S.
Performed the analysis: Lee JW, Bae JS, Kim JH, Cho HW, Ju HY, Yoo KH, Koo HH, Woo SY, Kim S.
Wrote the paper: Lee JW, Bae JS, Sung KW.
Acknowledgments
This work was supported by grants from the National Research Foundation of Korea (NRF), which is funded by the Korea government (NRF-2016R1A2B1012908 and NRF-2018R1A2B600325313), and by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (No. 1520210). The biospecimens for this study were provided by Samsung Medical Center BioBank.
References
2. Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003; 3:203–16.

3. Hero B, Simon T, Spitz R, Ernestus K, Gnekow AK, Scheel-Walter HG, et al. Localized infant neuroblastomas often show spontaneous regression: results of the prospective trials NB95-S and NB97. J Clin Oncol. 2008; 26:1504–10.

4. Moen EL, Godley LA, Zhang W, Dolan ME. Pharmacogenomics of chemotherapeutic susceptibility and toxicity. Genome Med. 2012; 4:90.

5. Roden DM, McLeod HL, Relling MV, Williams MS, Mensah GA, Peterson JF, et al. Pharmacogenomics. Lancet. 2019; 394:521–32.

6. Lennard L, Lilleyman JS, Van Loon J, Weinshilboum RM. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. 1990; 336:225–9.

7. Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015; 33:1235–42.

8. Bagatell R, McHugh K, Naranjo A, Van Ryn C, Kirby C, Brock P, et al. Assessment of primary site response in children with high-risk neuroblastoma: an international multicenter study. J Clin Oncol. 2016; 34:740–6.

9. Lee JW, Lee S, Cho HW, Ma Y, Yoo KH, Sung KW, et al. Incorporation of high-dose (131)I-metaiodobenzylguanidine treatment into tandem high-dose chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma: results of the SMC NB-2009 study. J Hematol Oncol. 2017; 10:108.

10. Sung KW, Son MH, Lee SH, Yoo KH, Koo HH, Kim JY, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation in patients with high-risk neuroblastoma: results of SMC NB-2004 study. Bone Marrow Transplant. 2013; 48:68–73.

11. Laska E, Meisner M, Wanderling J. A maximally selected test of symmetry about zero. Stat Med. 2012; 31:3178–91.

12. Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007; 13:559–65.

13. Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010; 26:2336–7.

14. Kishida Y, Kawahara M, Teramukai S, Kubota K, Komuta K, Minato K, et al. Chemotherapy-induced neutropenia as a prognostic factor in advanced non-small-cell lung cancer: results from Japan Multinational Trial Organization LC00-03. Br J Cancer. 2009; 101:1537–42.

15. Ma RM, Chen CZ, Zhang W, You J, Huang DP, Guo GL. Prognostic value of chemotherapy-induced neutropenia at the first cycle in invasive breast cancer. Medicine (Baltimore). 2016; 95:e3240.

16. Kasi PM, Kotani D, Cecchini M, Shitara K, Ohtsu A, Ramanathan RK, et al. Chemotherapy induced neutropenia at 1-month mark is a predictor of overall survival in patients receiving TAS-102 for refractory metastatic colorectal cancer: a cohort study. BMC Cancer. 2016; 16:467.

17. Chen Y, Wang Y, Shi Y, Dai G. Timing of chemotherapy-induced neutropenia predicts prognosis in metastatic colon cancer patients: a retrospective study in mFOLFOX6-treated patients. BMC Cancer. 2017; 17:242.

18. Sung L, Aplenc R, Alonzo TA, Gerbing RB, Wang YC, Meshinchi S, et al. Association between prolonged neutropenia and reduced relapse risk in pediatric AML: a report from the children’s oncology group. Int J Cancer. 2016; 139:1930–5.

19. Alexander S, Pole JD, Gibson P, Lee M, Hesser T, Chi SN, et al. Classification of treatment-related mortality in children with cancer: a systematic assessment. Lancet Oncol. 2015; 16:e604–10.

20. White GR, Varley JM, Heighway J. Genomic structure and expression profile of LPHH1, a 7TM gene variably expressed in breast cancer cell lines. Biochim Biophys Acta. 2000; 1491:75–92.

Fig. 1
Survival outcomes according to the absolute neutrophil count (ANC) group. Cumulative incidence of progression/treatment-related mortality, event-free survival, and overall survival based on an ANC cutoff value of 32.5/μL in all patients (A), in patients aged ≥ 2 years (B), and in high-risk patients (C).
Fig. 2
Tumor response according to the absolute neutrophil count (ANC) group. The percentage of residual tumor volume at the first response evaluation in all patients (A), in high-risk patients (B), and in patients with undifferentiated (UD) or poorly differentiated (PD) neuroblastoma (C).
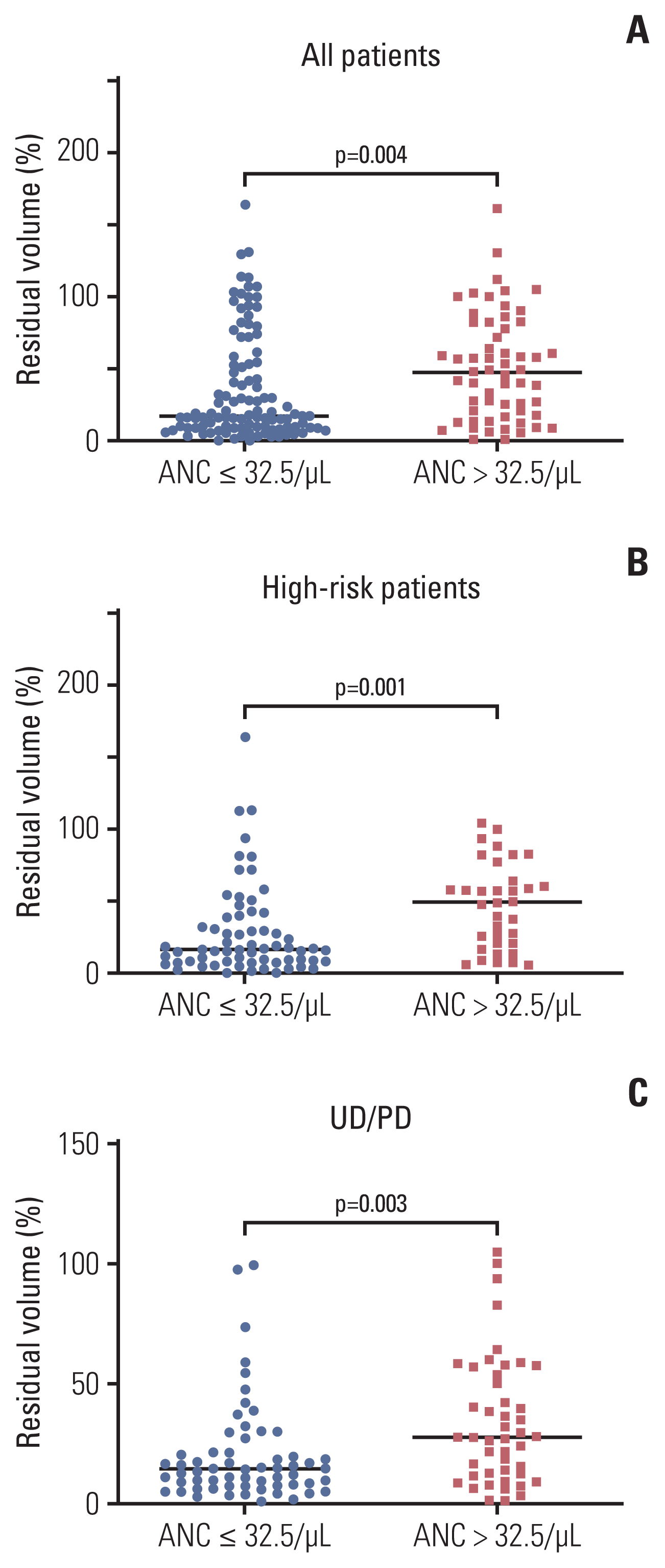
Fig. 3
Cumulative incidence of treatment-related mortality (TRM) according to the absolute neutrophil count (ANC) group. An ANC of 51.0/μL was selected as an optimal cutoff point for the cumulative incidence of TRM, and patients in the ANC > 51.0/μL group showed a lower 5-year cumulative incidence of TRM than those in the ANC ≤ 51.0/μL group (1.3%±0.02% vs. 9.4%±0.04%, respectively) in all patients (A) and in high-risk patients (B).
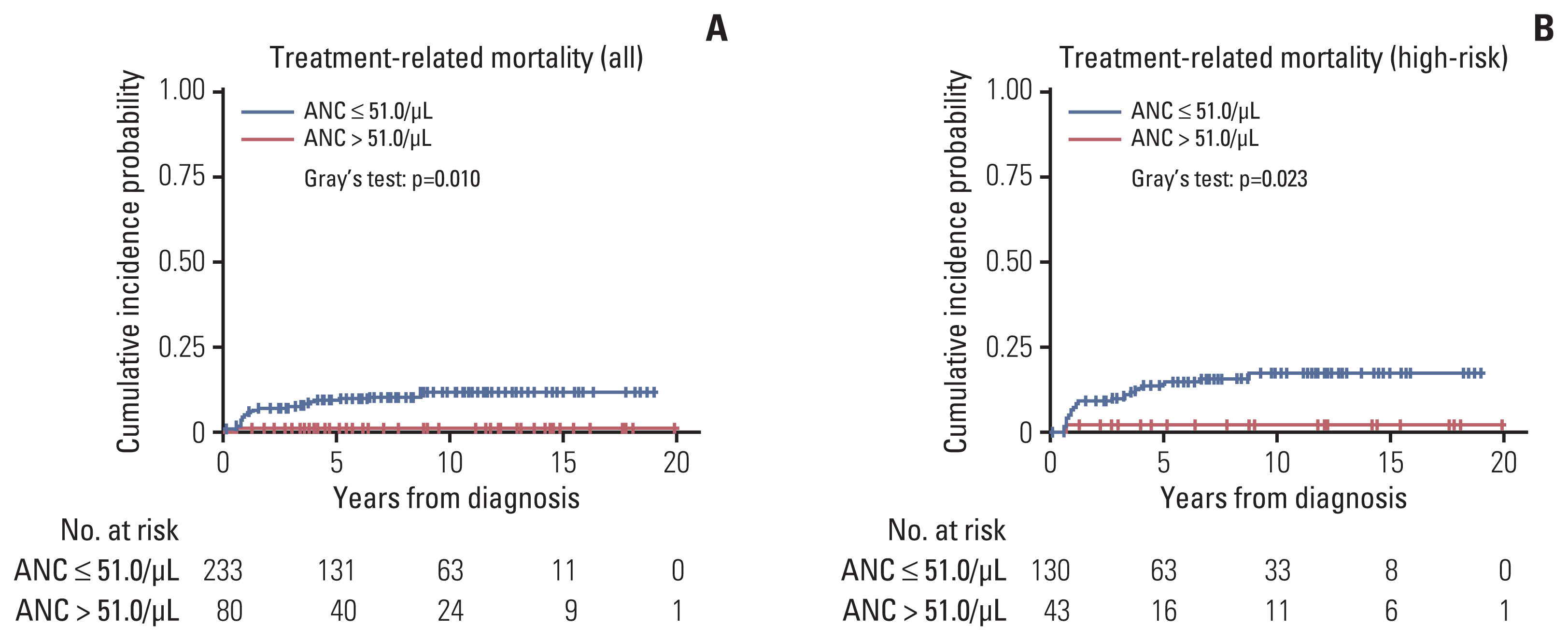
Fig. 4
Manhattan plot of the genome-wide association study. (A) Results of the genome-wide association analyses of common single- nucleotide polymorphisms (SNPs) (minor allele frequency > 0.05) associated with the absolute neutrophil count represented as a Manhattan plot. The X-axis represents the SNP markers on each chromosome. (B) Regional association plots at the RPTN locus. Regional association plots, including both genotypes and SNPs of the LPHN2, were generated using LocusZoom within 400 kb.
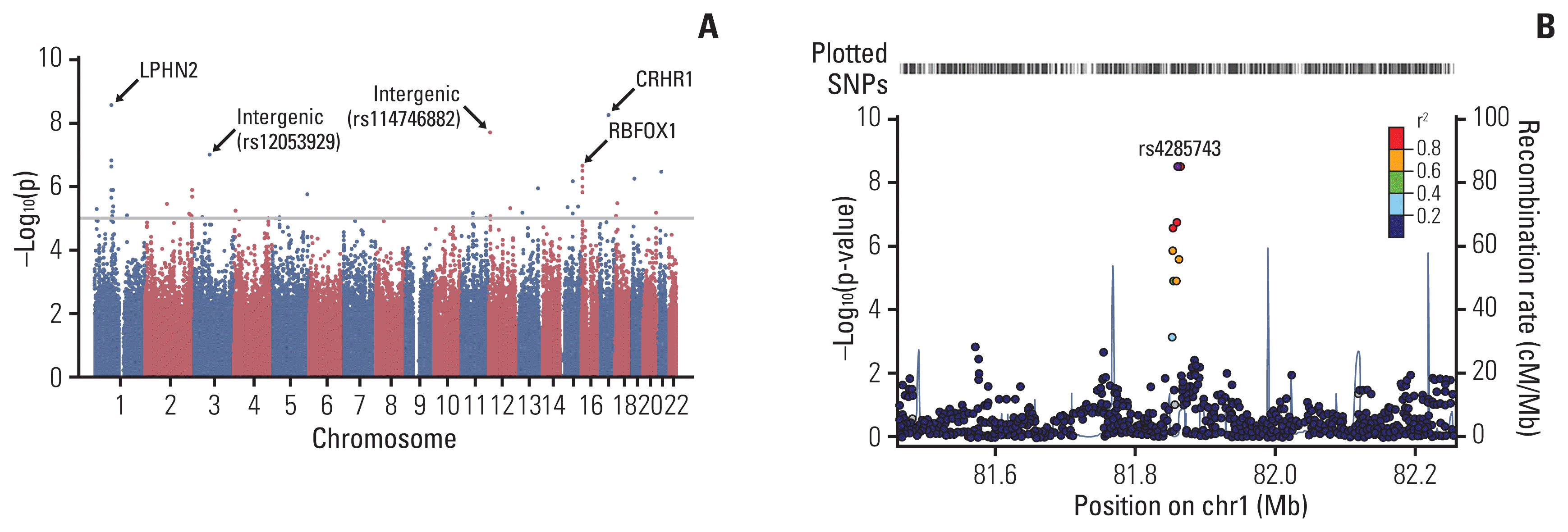
Table 1
Patient characteristics
Table 2
Multivariate analysis for the cumulative incidence of progression




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download