Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide [
1]. The occurrence of metastasis in CRC is considered a highly fatal course [
2]. However, the theory of oligometastases suggest that a reduced number of metastases represent a transitional state between localized and diffuse disease, and local control of the metastases might lead to improved outcomes [
3,
4]. In fact, under favorable settings of oligometastases, median survival may surpass 5 years in patients with solitary lung or liver metastasis [
5,
6]. On the other hand, there are some patients who rapidly recurred after potentially curative metastasectomy [
7]. To date, there are no established guidelines for the selection of patients who require aggressive treatments to maximize their long-term survival and those for whom aggressive treatments may be harmful.
As clinical risk factors, the local stage of primary tumor, synchronicity of metastases, tumor marker level, number and size of metastases, and disease-free interval were reported to be significantly associated with tumor recurrence after metastasectomy [
8,
9]. However, these risk factors are hardly used in the clinical practice [
10]. Some observations support that oligometastatic prognosis in CRC mainly relies on biological drivers rather than on clinical risk factors. Despite constant progress in imaging modalities, systemic treatments, and surgical techniques, the proportion of patients cured after metastasectomy has not significantly improved over recent decades [
7].
Several mutational molecular markers identified in CRC include microsatellite instability,
BRAF, and
KRAS/
NRAS, which confer poorer outcomes [
11]. However, most of these studies are based on primary cancers, and little is known regarding the molecular profile of metastasis and its relation to clinical outcomes in oligometastatic cancers [
12]. Moreover, emerging evidence of both intra- and inter-tumor heterogeneities in several solid tumor types have raised concerns that the molecular profiling of primary tumors may not be representative of metastatic disease [
13-
15]. Intra-tumor heterogeneity refers to differences within a single tumor or between primary and metastatic tumors, and inter-tumor heterogeneity refers to differences between patients [
16]. Further studies are needed because the tumor heterogeneity can confuse the application of biomarkers that may predict treatment response or prognosis.
In this study, we aimed to identify the concordant or discordant genomic profiles between primary and matched metastatic tumors from patients with CRC undergoing metastasectomy and we attempted to classify genomic groups according to genomic discrepancy patterns and identify candidate genomic patterns that could affect survival after metastasectomy.
Discussion
Advances in next-generation sequencing technology in recent decades make that knowledge of the biological drivers of cancer will lead to personalized cancer treatment [
13,
17]. Indeed, the accumulation of molecular alterations of these key driver genes plays a crucial role in the tumorigenesis and progression of CRC. However, it remains controversial, as previous studies usually rely on a limited sample of cancer tissue that cannot represent heterogeneity between and within patients [
18]. This study is an integrated analysis of intra-tumor and inter-tumor heterogeneity. We found the overall intra-tumor heterogeneity by comparing primary and metastatic tumors, and we yielded the genomic groups based on the inter-tumor heterogeneity between patients. We also obtained some notable correlations between paired genes and metastatic sites by considering the tumor heterogeneity.
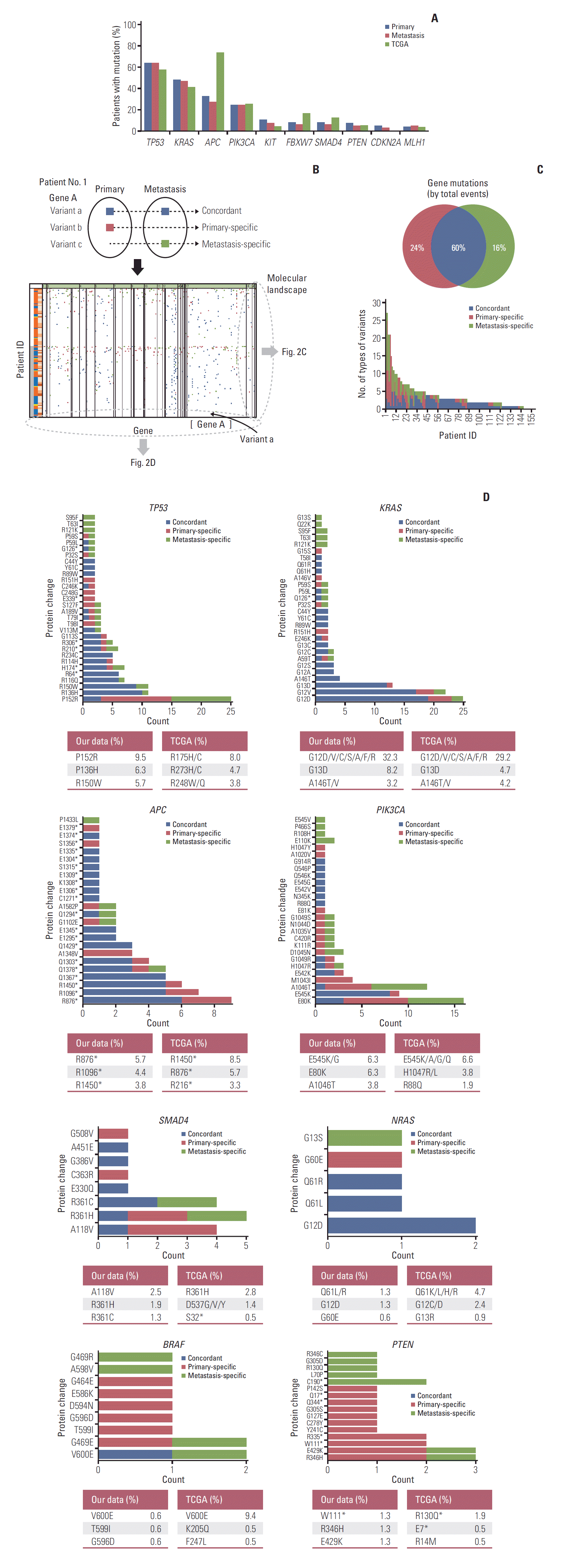
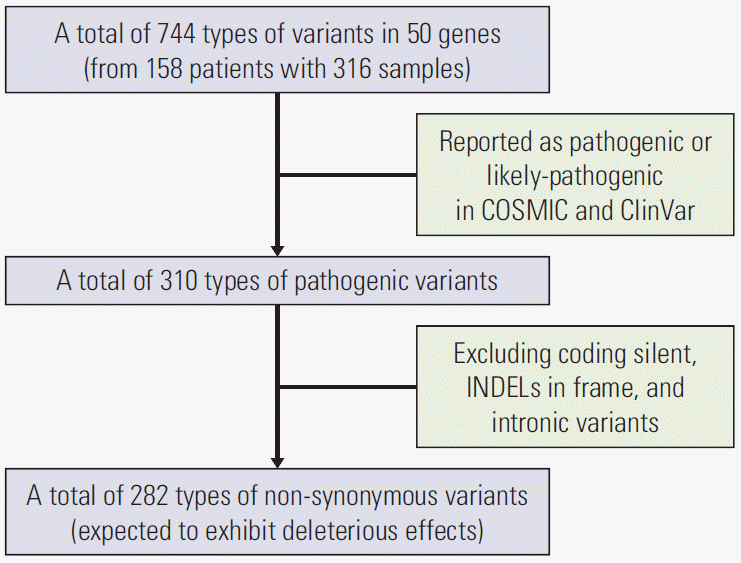
First, in demographics, there was a lower prevalence of
APC genes in the Korean population compared with TCGA data. The frequency of
APC mutations between our data and TCGA data may vary owing to the definition of the mutations. TCGA data included truncating mutations (putative driver) and missense mutations (unknown significance). However, since our data only included pathogenic or likely pathogenic variants classified in the COSMIC or ClinVar databases, the frequencies of
APC gene were reduced by 32.3% and 27.2% for primary and metastatic tumors. In our data, when we extend our mutation definition as total variants, the frequencies of
APC gene were up to 81% and 72.8% for primary and metastatic tumors, respectively. The majority of
APC gene mutations are deletions or insertions or nonsense mutations, which result in the formation of a truncated
APC protein [
19]. However, in our data, only 36.8% of total variants have been classified as either pathogenic or likely pathogenic variants in the clinical significance value of ClinVar or COSMIC. Consistent with our data, Inra et al. [
20] reported that the frequencies of pathogenic
APC variants in 8,676 personal and/or family history of colorectal polyps and/or CRC were 17.5% overall, 25.2% in Asians, and 15.5% in Caucasians. As more than 50% of mutations in
APC gene have uncertain or unknown clinical significance, further studies are needed to evaluate the clinical significance value of
APC mutation and to accumulate more updated data.
Next, we showed there was an average of 40% genomic discrepancy between primary and matched metastases. More discordant variants were caused by variant presence in the primary CRC tumors and absence in the metastases. The ratio of primary-specific variants to metastasis-specific variants was 3:2. Indeed, primary CRCs could include a large cohort of passenger mutations, of which approximately 71.4% occur in the matching metastases. In previous studies, Brannon et al. have reported a 100% concordance for
KRAS,
NRAS, and
BRAF between primary and metastatic CRCs [
21]. However, in their study, the primary tumor and metastasis were simultaneously resectioned in 75% of patients. In addition, 43% of patients were chemotherapy-naive prior to resection. In our study, there is a time interval between primary and metastatic tumor resections (55.1% of patients exhibit metachronous metastasis; mean time interval between resections of metachronous metastasis is 24.9 months), and 86.1% of our patients were administered chemotherapy after primary resection. Chemotherapy after primary resection can cause genetic changes through cancer cell necrosis. For these reasons, discordances might be more in this study compared with that in the study by Brannon et al. Furthermore, we identified the frequency and position of each variant of key genes for concordant, primary-specific, and metastasis-specific variants. The portion of concordant variants was large in
TP53,
KRAS,
APC, and
NRAS, which are early mutations in CRC. In contrast, the portion of discordant mutations was large in
PIK3CA,
SMAD4,
BRAF, and
PTEN, which are known as later mutations. This can be explained through heterogeneous clonal evolution [
22].
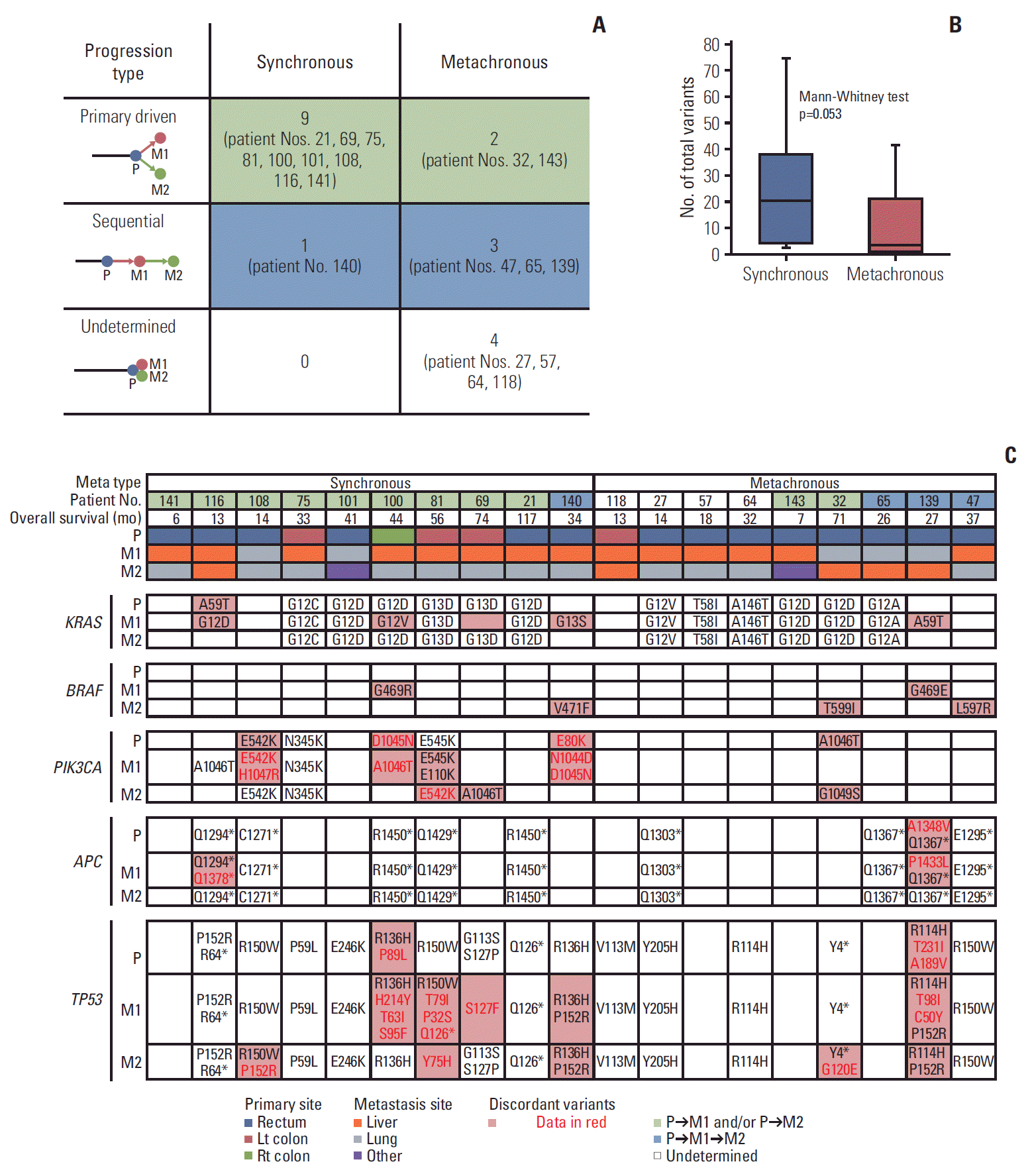
In terms of clonal evolution, additional intra-tumor heterogeneity was identified in 19 patients with two serial metastatic tumors. Interestingly, in synchronous metastasis, primary and metastatic tumors simultaneously showed different biology, as in the Big Bang model [
23]. However, when M2 occurs, the genetic origin of M1 is followed by P, instead of sequential M1, which suggests the high effect of primary tumors on synchronous metastasis; it appears born-to-bebad. On the other hand, in metachronous metastasis, biology was similar between primary and metastatic tumors, and it developed in a more sequential way according to the accumulation of mutations. In CRC, the driver gene of the primary tumor plays a pivotal role in most cases. However, in some patients, changes in metastasis-specific genes can cause clonal evolution in a different direction than primary cancer, as demonstrated by patients 140 and 139, i.e., both showed simultaneous metastasis.
TP53 P152R played a pivotal role in these two patients (
S8 Fig.). Further research is needed on metastasis-specific mutations and their mechanisms, which play a pivotal role in clone evolution.
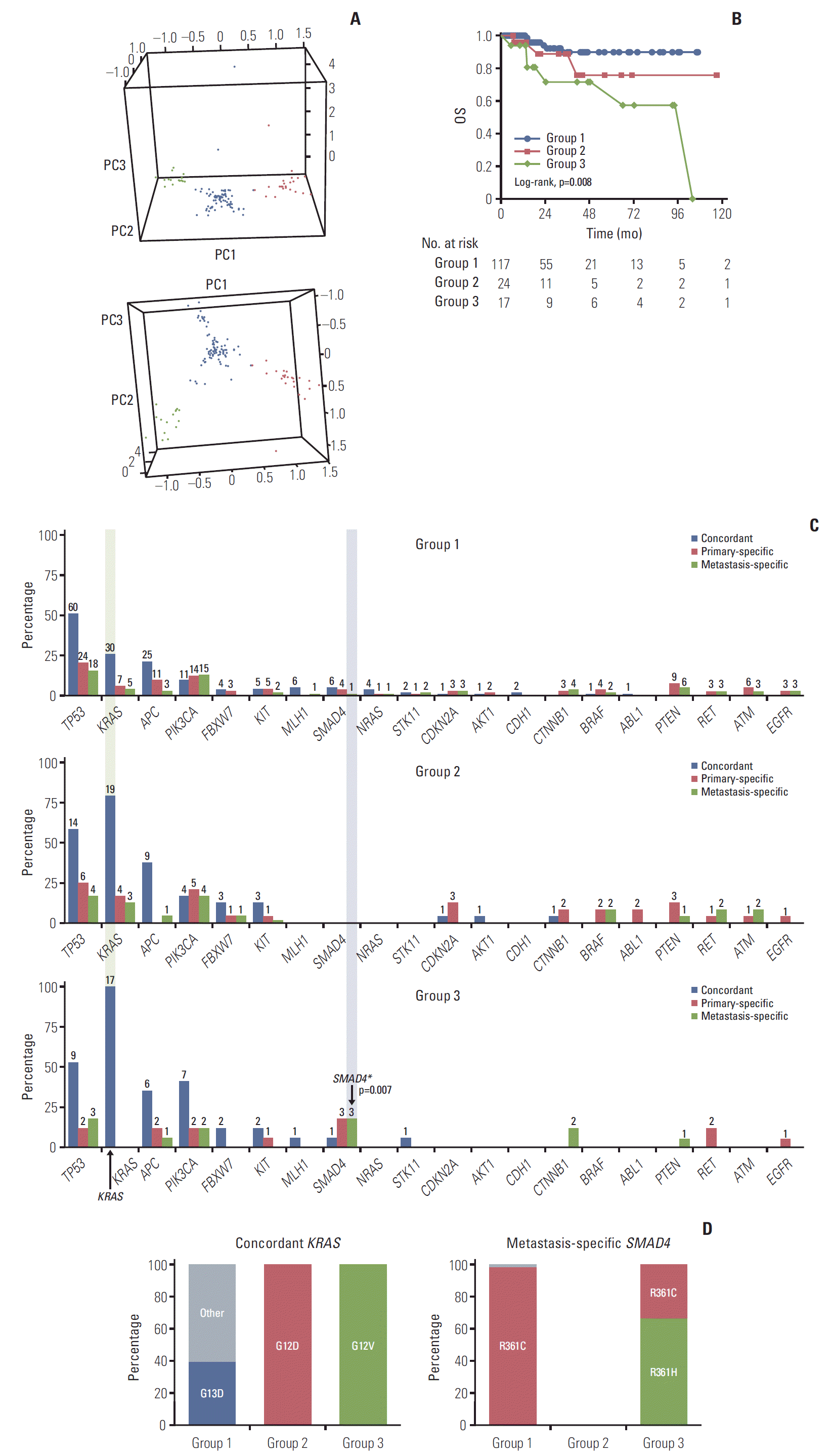
We used PCA and clustering to generate genomic groups based on inter-tumor heterogeneity that reflects intra-tumor heterogeneity. The genomic groups classified based on genomic discrepancy patterns showed significant differences in OS. Genomic groups remained significant even after correcting for other clinical risk factors, thus, suggesting that biological factors strongly influence the clinical outcome. Concordant
KRAS G12V and metastasis-specific
SMAD4 were identified as characteristics of the poor OS group (group 3).
KRAS mutations occur early during the progression from colorectal adenoma to malignant carcinoma. Of note, the Ras-Raf-MEK-ERK and phosphoinositide 3-kinase/AKT pathways are strongly interconnected. Activation of this network by muta-tion deregulates the survival, mobility, and proliferation of cells [
24]. In a recent study, specific mutations in
KRAS codon 12 were associated with shorter OS in patients with advanced and recurrent CRC, especially in G12V [
25]. This result is consistent with our results. Moreover, we found that no newly acquired metastasis-specific
KRAS mutations were observed in group 3. Groups 1 and 2 accounted for more than 20% of metastasis-specific
KRAS G12V (
S6 Fig.). This suggests that concordant
KRAS G12V had a more significant effect on survival than newly acquired
KRAS G12V in metastasis.
As the pivotal factor of the transforming growth factor β pathway, which regulates tumorigenesis and tumor progression,
SMAD4 mutations were reported to be present in 2.1%-20.0% of CRC [
26]. In a meta-analysis, Huang et al. [
18] reported that patients with CRC who had a
KRAS mutation (combined odds ratio [OR], 1.29; 95% CI, 1.13 to 1.47) or
SMAD4 mutation (combined OR, 2.04; 95% CI, 1.41 to 2.95) were at a higher risk of distant metastasis. No significant association was found between mutations of
APC or
PIK3CA and CRC metastasis [
18]. Considering that the occurrence of distant metastasis is associated with poor prognosis, this is consistent with our results. However, while the results of previous studies were obtained from an OR of each gene, our study considered several genes concurrently. Considering these results, patients who had
KRAS G12V mutation in the primary tissue at the time of diagnosis should confirm the genetic changes or mutations present in the metastatic tissue when they have to decide the treatment directions or to predict the prognosis. However, even in patients who did not have this mutation, it is necessary to confirm those of metastatic tissues before determining the treatment directions as it helps in identifying mutations with poor prognosis, such as
SMAD4.
In other aspects of primary and metastasis research, Joung et al. [
27] classified oligo-clones and multi-clones based on the number of subclones in primary and metastatic tumors using a hierarchical Bayesian clustering model [
27]. There was a significant difference in the DFS between the oligoclone and multi-clone groups, but a difference in OS was not proven. This result differed from our findings, because they quantified heterogeneity that did not reflect the types of genetic mutations. We assessed all the components, including the discrepancy pattern between primary and metastatic tumors and types of genetic variants. Overall, these findings suggest that the degree of heterogeneity is related to DFS, but it is the genetic mutations that affect the OS.
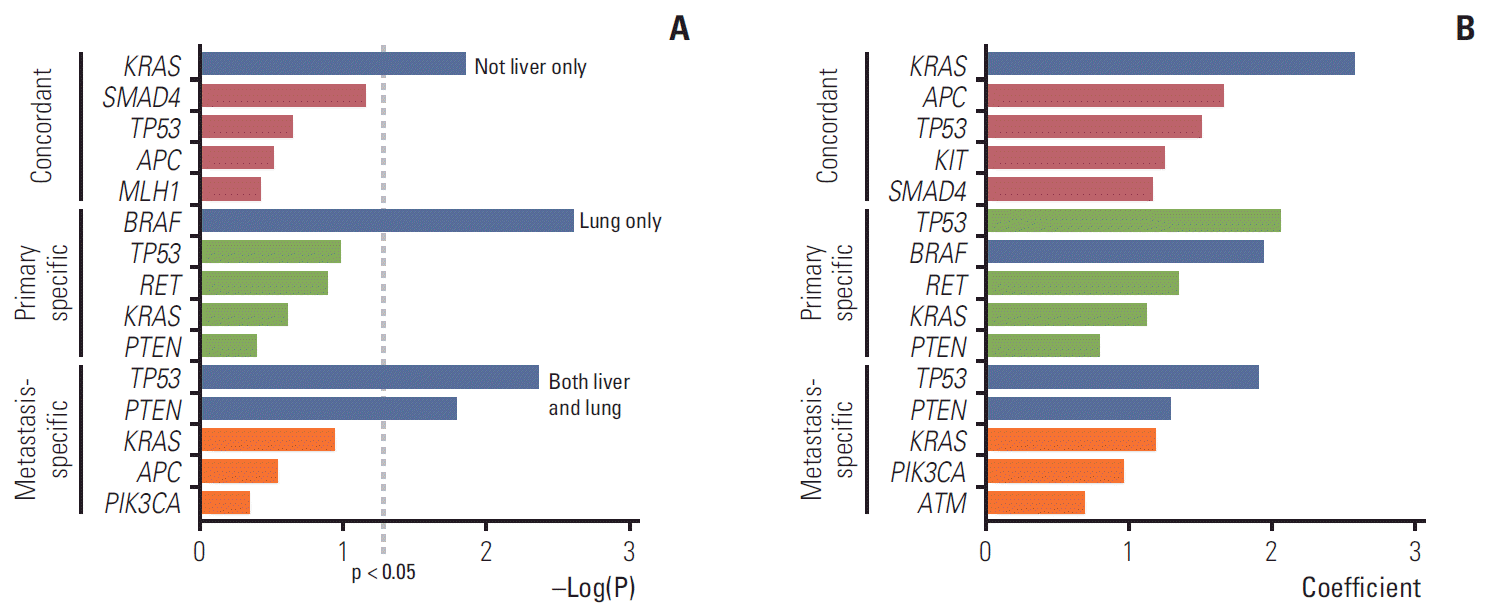
To the best of our knowledge, the present study is the first to document a preference for metastatic sites based on paired genes. These findings correlated well with our survival data. Concordant
KRAS that occupied a high proportion of group 3 tended to disseminate and were not confined to one organ. When a random forest model was applied, the metastatic sites were predicted by the conditions of several genes frequently found in CRC. Datta et al. [
28] reported that metastatic CRC tumors harboring co-occurring
RAS/
BRAF and
TP53 alterations were significantly more likely to involve extrahepatic metastatic sites compared with tumors that were
RAS/
BRAF-altered or
TP53-altered only. This analysis considered the synergistic effects of the three genes, and the results for key genes were similar to our findings. However, our study considered the conditional effects of 50 genes that can be detected. The results showed extrahepatic metastasis in concordant
KRAS and metastasis-specific
TP53 and
PTEN. Primary-specific
BRAF was more likely to be associated with lung metastasis. Fidler [
29] insisted that clone survival was dependent on the surrounding environment, such as the liver and lungs, by the seed-soil hypothesis. Further research is required on the mechanisms of clonal selection according to metastatic sites and their interactions with other genetic mutations.
The present study had several limitations. First, we included only deleterious non-synonymous variants. In recent studies, synonymous variants with deleterious effects have been reported [
30]. For instance, synonymous variants may create a new splicing site or obliterate an existing one, thus, turning an exonic sequence into an intron, or vice versa, which results in the production of a different polypeptide. However, in our study, most of the synonymous variants had a high frequency of silent variants and few had no exact function or effect. Second, the criteria for oligometastasis was not clear. Although, we included patients with oligometastases occurring in resectable CRC, the study population may be a heterogeneous group depending on whether it is a debulking operation or staged operation. However, this heterogeneity was corrected for by the clinical factors, including metastatic types and sum of the diameter of metastasis lesions at the time of metastasectomy. Third, the present study is a model for the current data set. This model may have an overfitting problem and is only significant in this enrolled patient group; therefore, validation with larger sample sizes is required. Fourth, compared with the average number of deleterious non-synonymous variants per patient (4.0), nine patients had more than 10 deleterious non-synonymous variants. Five out of nine patients were confirmed low microsatellite instability/microsatellite stable and the microsatellite instability status of four could not be identified. One case out of nine corresponded to mucinous carcinoma. However, seven of the nine patients were in group 1 and two patients were in group 2. Although it was not possible to determine the overall MSI status, we could rule out the possibility that group 3 with poor prognosis was biased to patients with a high number of variants.
In conclusion, driver gene mutations were mostly concordant; however, discordant or metastasis-specific mutations were present. Clinically, the concordant driver genetic changes, especially in KRAS G12V mutations, with additional metastasis-specific variants, especially in SMAD4 mutations, can predict poor prognosis in patients with CRC. These findings suggest that we must consider the biology of the metastatic tumor compared with the primary tumor, as this can help guide treatment and predict the survival of patients with oligometastatic CRCs.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download