Abstract
Purpose
The purpose of this study was to investigate the diagnostic value of magnetic resonance imaging (MRI)–ultrasound (US) fusion transperineal targeted biopsy (FTB) and fusion template systematic biopsy (FSB) for prostate cancer (PCa) and clinically significant prostate cancer (csPCa) (intermediate/high grade [Gleason score ≥ 3+4]) based on bi-parametric MRI (bpMRI).
Materials and methods
Retrospectively, we analyzed 300 patients with elevated prostate-specific antigen (≥ 4.0 ng/mL) and/or abnormal findings in a digital rectal examination at the Korea University Hospital. All 300 men underwent bpMRI-US fusion transperineal FTB and FSB in the period from April 2017 to March 2019.
Results
PCas were detected in 158 of 300 men (52.7%), and the prevalence of csPCa was 34.0%. CsPCas were detected in 12 of 102 (11.8%) with Prostate Imaging-Reporting and Data System (PI-RADS) 3, 42 of 92 (45.7%) with PI-RADS 4, respectively; and 45 of 62 (72.6%) men with PI-RADS 5, respectively. BpMRI showed a sensitivity of 95.1% and negative predictive value of 89.6% for csPCa. FTB detected additional csPCa in 33 men (12.9%) compared to FSB. Compared to FTB, FSB detected additional csPCa in 10 men (3.9%).
Conclusion
BpMRI-US FTB and FSB improved detection of PCa and csPCa. The accuracy of bi-parametric MRI is comparable with that of multi-parametric MRI. Further, it is rapid, simpler, cheaper, and no side effects of contrast media. Therefore, it is expected that bpMRI-US transperineal FTB and FSB could be a good alternative to conventional US-guided transrectal biopsy, which is the current gold standard.
Multi-parametric magnetic resonance imaging (mpMRI) has excellent sensitivity for prostate cancer (PCa) detection, and targeted biopsy using mpMRI findings has additional diagnostic value for detecting PCa [1-5]. In addition, compelling evidence has been reported for the role of mpMRI in patients with a raised prostate-specific antigen (PSA) level [3,6]. With the development of mpMRI interpretation, such as the Prostate Imaging-Reporting and Data System (PI-RADS) [7-9], efforts are being made to increase the detection rate of clinically significant prostate cancer (csPCa) (intermediate/high grade [Gleason score ≥ 3+4]) through targeted biopsy and to reduce overtreatments by lowering the detection rate of insignificant PCa [Gleason score=3+3]) [10]. PCa shows multifocality in up to 60%-90% of the cases [11] while csPCa are missed on mpMRI in up to 24%-28% of the cases [12]. To determine the direction of management and to prevent overtreatment, accurate diagnosis through evaluation of the whole prostate gland, including invisible lesions on magnetic resonance imaging (MRI) is essential [13,14]. Therefore, most guidelines recommend pre-biopsy mpMRI if there is a negative biopsy history but persistent suspicion of PCa and to combine targeted and systematic biopsy in biopsynaïve patients if there is a positive finding on mpMRI [15,16]. Further, MRI-ultrasound (US) fusion biopsy is being a widely used technique.
MpMRI consists of T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and dynamic contrast-enhanced (DCE) imaging. MpMRI is time-consuming (approximately 40 minutes), expensive, and requires intravenous administration of contrast media, which could have side effects. Immobilization of the patient during image acquisition is required for accurate comparison of each mpMRI scan sequence [17]. However, bi-parametic MRI (bpMRI) is rapid (approximately 15 minutes) and simpler and uses fewer scan sequences and no intravenous contrast media [18]. BpMRI could minimize the limitations while retaining sufficient diagnostic value of mpMRI. However, the accuracy and role of bpMRI to detect PCa and csPCa remains unclear. Here, we report the diagnostic accuracy and value of MRI-US fusion transperineal targeted and template systematic biopsy (FTSB) for PCa and csPCa detection based on bpMRI with PI-RADS ver. 2.0.
Retrospectively, we analyzed 300 men with a raised PSA level (≥ 4.0 ng/mL) and/or abnormal findings on a digital rectal examination at the Korea University Hospital. All 300 men underwent MRI-US transperineal FTSB based on bpMRI in the period from April 2017 to March 2019.
Before biopsy, all patients underwent bpMRI using a 3.0-T scanner (Siemens Medical System, Erlangen, Germany) with T1 weighted image, biplanar T2WI, DWI, and the apparent diffusion coefficient without the DCE imaging sequence. Regions on the bpMRI were marked by three dedicated uroradiologists based on the PI-RADS ver. 2.0 [7]. We set regions with PI-RADS ≥ 3 on bpMRI as the regions of interest (ROI) and used them as targeted regions. When ROI was not present on bpMRI, only the transperineal template mapping systematic biopsy was performed using the MRI-US fusion technique. The elastic image registration type of the MRI-US fusion technique using a mechanical position encoder and robotic articulated arm system (D&K Technologies GmbH, Barum, Germany) was used, in the same session, targeted biopsies and systematic biopsies (using the modified Barzell-template) were performed based on the prostate size. The ROI lesion was not intentionally avoided during the systematic biopsy but rather performed using a routine method via template prostate mapping biopsies in a routine manner.
All biopsies were performed under monitored anesthesia care (MAC) or general anesthesia (GA). To avoid complications associated with GA, we preferred MAC anesthesia with propofol for sedation and fentanyl for pain control, without muscle relaxants. GA was performed based on the discretion of an anesthesiologist depending on the patient’s condition. The biopsies were performed by two urologists. Definition of csPCa was the presence of Gleason pattern 4, Gleason score ≥ 7 (3+4).
Based on the Clavien-Dindo system, complications were classified into four grades. Statistical analyses were performed using the SAS software ver. 9.4 (SAS Institute Inc., Cary, NC) and R software 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
The mean age was 66.6 years. The mean PSA level was 11.3 ng/mL, and the mean PSA density was 0.33. There were 215 biopsy-naïve men and 85 repeat-biopsy men. There were no significant differences among the clinical parameters based on the prior history of biopsy. The average time for bpMRI-US FTSB was 29.3 minutes. The conversion rate from MAC to GA was 31% (93 out of a total 300 patients). Fusion transperineal targeted biopsy (FTB) was performed with an average of 5.3 core biopsies (maximal up to 10 cores) per ROI, additional 14-20 template prostate mapping systematic biopsies were performed based on the prostate size and the average cores of fusion template systematic biopsy (FSB) were 18.6. Table 1 lists the characteristics.
There were 44 out of 300 men with no ROI, 256 out of 300 men with ROIs (PI-RADS ≥ 3). A total of 301 ROIs were analyzed among the ROI detected 256 men (1.18 per person). The number of men with PI-RADS 0-2, 3, 4, and 5 was 44 (14.6%), 102 (34.0%), 92 (30.6%), and 62 (20.6%), respectively.
PCa were detected in 158 out of 300 men (52.7%), and csPCa [Gleason score ≥ 7 (3+4)] were detectected in 102 of 300 men (34.0%) on bpMRI-US transperineal FTSB. The detection rate of PCa and csPCa were 54.3% and 34.8% with the FTB, and 45.3% and 25.8% with the FSB, respectively.
PCa and csPCa were detected in 11 of 44 (25.0%) and in three of 44 (6.8%) patients with PI-RADS 0-2 (no ROIs), in 26 of 102 (25.5%) and in 12 of 102 (11.8%) with PI-RADS 3, in 65 of 92 (70.7%) and in 42 of 92 (45.7%) with PI-RADS 4, in 56 of 62 (90.3%) and in 45 of 62 (72.6%) with PI-RADS 5, respectively (Table 2).
FTB detected additional prostate cancers in 31 men (12.1%) in whom PCa was not detected with systematic biopsy and additional csPCa in 33 men (12.9%) in whom no cancers or insignificant PCa (gleason score 6 (3+3)) were detected with FSB. Compared to FTB, FSB detected additional prostate cancers in eight men (3.1%) in whom no cancers were detected with FTB and additional clinically significant prostate cancers in 10 men (3.9%) in whom no cancers or insignificant prostate cancers were detected with FTB (Fig. 1).
The detection rate of PCa and csPCa was 54.0% and 34.0% in biopsy-naïve men, respectively; 49.4% and 34.1% in men with history of prior biopsy, respectively. There were no significant differences of detection rate of PCa and csPCa based on history of prior biopsy (Table 3).
BpMRI showed a sensitivity of 92.1% (95% confidence interval [CI], 86.9 to 95.7), specificity of 19.7% (95% CI, 14.1 to 26.3), positive predictive value (PPV) of 51.5% (95% CI, 45.7 to 57.4) and negative predictive value (NPV) of 72.9 % (95% CI, 58.2 to 84.7) for PCa, respectively; a sensitivity of 95.1% (95% CI, 88.9 to 98.4), specificity of 17.8% (95% CI, 13.2 to 23.3), PPV of 32.9% (95% CI, 27.6 to 38.6), and NPV of 89.6% (95% CI, 77.3 to 96.5) for csPCa, respectively (Table 4, S1 Table).
The number of complications for MRI-US transperineal FTSB was in 94 of 300 (31.3%), all of which were minor complications (Clavien grade 1-2). The most frequent complications were mild hematuria (71.2%) and urinary retention (28.1%). There were no major complications (Clavien grade 3-4) that required intervention or intensive care unit admission (Table 5).
Many previous studies have reported the superiority of targeted and systematic biopsy based on mpMRI compared to US-guided transrectal biopsy [15]. The transition from conventional US-guided transrectal biopsy to the combination of targeted biopsy and systematic biopsy is inevitable [16].
There are three questions to consider when performing prostate biopsy for accurate PCa diagnosis.
First, should MRI be performed in all men considering the prostate biopsy?
If yes, what kind of MRI (mpMRI or bpMRI) should be chosen?
As for the diagnostic value, the targeted biopsy reported a detection rate of 38% for csPCa and 12% additional diagnostic value to systematic biopsy in the PRECISION study based on mpMRI [2]. Further, in the meta-analysis, MRI-FIRST trial, and 4M trial, additional values of targeted biopsy were reported to be from 3.2% to 6.0% [15,16]. Ahmed et al. [1] reported a sensitivity, specificity, PPV and NPV of 88%, 45% 65% and 76% for PCa and 93%, 41%, 51% and 90% for csPCa, respectively. Therefore, MRI should be performed in all men considering the prostate biopsy. Although mpMRI with the DCE mode plays a significant role in detecting PCa based on the PI-RADS [9,19], as shown in this study, bpMRI showed comparable sensitivity and NPV for detection of csPCa (95.1% and 89.6%, respectively) compare to mpMRI (93.0% and 89.0%, respectively) [1]. Further, bpMRI is rapid, simpler, cheaper and no side effects associated with the contrast media. BpMRI is expected to overcome the drawbacks of mpMRI and could be a good alternative.
Second, what type of targeted biopsy should be performed?
The targeted biopsy technique using MRI findings includes in-bore MRI targeted biopsy, cognitive biopsy, and MRIUS fusion biopsy [14]. In many prior studies, there were no differences in PCa and csPCa detection based on the biopsy technique and route [20]. Technique and route selections are possible based on the pros and cons of modalities and clinical condition. In cases of in-bore MRI targeted biopsy, real-time biopsy is impossible due to temporal and spatial constraints. Therefore, there seems to be a real barrier to clinical application. Cognitive biopsy has a disadvantage of high dependency on the operator's ability in judging the targeted lesion and matching to US. Further, unnecessary needling is needed to confirm the location of the biopsy needle for targeting and mapping [21,22]. In contrast, the MRI-US fusion technique does not depend on the ability of the surgeon, and it can reduce unnecessary needling with the navigating system and template of the MRI-US fusion technique.
Third, can we omit the systematic biopsy?
What kind of method should be used if systematic biopsy should be performed?
The detection rate of csPCa is increased and that of insignificant cancers is decreased in patients undergoing targeted biopsy alone compared to systematic biopsy [23]. However, 10 men (3.9%) with csPCa were missed compared to targeted biopsy alone. Further, three in 44 men (6.8%) with csPCa were detected in only systematic biopsy when there was not visible lesion on MRI. Therefore, it should not be overlooked [14,24]. To determine the appropriate treatment, whole gland evaluation is required [25,26]. Compared to the whole mount gland specimen after prostatectomy, there were missed csPCa with transrectal biopsy. Transperineal biopsy has the advantage of evaluation of the whole gland through accurate mapping of invisible lesions on MRI [23] and bowel preparation is no longer required. There are no differences in the minor complications [27]. In this study, there were not major complications (Clavien grade 3-4) that required intervention or intensive care unit admission. Therefore, systematic prostate biopsy cannot be omitted based on the results of MRI, and transperineal template biopsy is a good choice for systematic biopsy [13].
This is a retrospective study, resulting in inherent limitation of study design. However, this study was a single arm study that focused on the diagnostic values of bpMRI transperineal FTSB. The results of this study showed that the performance of bpMRI may be comparable to mpMRI and may also offer potential benefits of overcoming the disadvantages of mpMRI. However prospective trials are necessary to reach any definitive conclusion.
Another limitation lies in that FTSB cannot be performed in an out-patient setting because preparation and the need for GA is always uncertain. MAC anesthesia uses propofol (initiation 0.5 mg/kg, maintenance 25-75 μg/kg/min) for sedation, and remifentanil (initiation 0.5 μg/kg, maintenance 0.05-0.2 μg/kg) for pain control. Thirty-one percent (n=93) of patients converted to GA. The most common cause was insufficient sedation and pain control even after using an adequate initial dose of propofol and remifentanil resulting in pain and movement of the patient. Because close monitoring for MAC is needed to provide sufficient anesthesiologic control, the clinical response of the patients and resultant conversions to GA were greatly dependant on the experience of the anesthiologist. Thus, with an experienced anesthiologist, we believe that FTSB is feasible under MAC anesthesia
Furthermore, this study is that csPCa was defined based on biopsy results. Transperineal biopsy method showed relatively higher sensitivity and accuracy (77% and 53%, respectively) compare to traditional transrectal biopsy (53% and 59%, respectively) when correlating the results with radical prostatectomy specimens [23]. According to Hu et al. [28], transperineal template biopsy reflects the spatial distribution of PCa resulting in a higher accuracy (area under the curve [AUC] ≈ 0.90) compared to transrectal biopsy (AUC, 0.70-0.80). Therefore, the results of transperineal biopsy could reflect the significance of PCa well in comparison with transrectal biopsy.
FTB has shown superior performance for detection of PCa and clinically significant PCa. It provided an additional diagnostic value for detection of csPCa. There were missed csPCa in men without an apparent ROI (targeted) lesion on MRI. Therefore, the role of systematic biopsy should not be overlooked and the combination of targeted and systematic biopsy is essential. In this study, the results of bi-parametric MRI were comparable to those of multi-parametric MRI in terms of diagnostic accuracy, high sensitivity (95.1%) and NPV (89.6%) compare to mpMRI (93.0% and 89.0%, respectively). Furthermore, bpMRI had advantages over mpMRI in terms of time, cost, and side effects of contrast media.
The PCa detection rate for US-guided transrectal biopsy which is the current gold standard is reported to be 20%-30% [20]. The detection rate for targeted and systematic biopsies utilizing mpMRI in the PROMIS study are reported to be 53.0% and 40.0% for PCa and csPCa, respectively [1]. The PCa and csPCa detection rate for bpMRI-US transperineal FTSB are 52.7% and 38.7%, respectively. In comparison to transrectal biopsy, the PCa detection rate is higher for bpMRI-US transperineal FTSB and its results are also comparable to mpMRI. In addition, there were no major complications (Clavien grade 3-4) that required intervention or intensive care unit admission. Furthermore, bpMRI is not only rapid and simpler than mpMRI, but also showed promising results that are comparable to mpMRI in terms of accuracy. Therefore, it is expected that bpMRI-US transperineal FTSB could be a good alternative to conventional US-guided transrectal biopsy, which is the current gold standard.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
References
1. Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017; 389:815–22.

2. Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018; 378:1767–77.

3. Drost FH, Osses DF, Nieboer D, Steyerberg EW, Bangma CH, Roobol MJ, et al. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev. 2019; 4:CD012663.

4. Bonekamp D, Schelb P, Wiesenfarth M, Kuder TA, Deister F, Stenzinger A, et al. Histopathological to multiparametric MRI spatial mapping of extended systematic sextant and MR/TRUS-fusion-targeted biopsy of the prostate. Eur Radiol. 2019; 29:1820–30.

5. Radtke JP, Schwab C, Wolf MB, Freitag MT, Alt CD, Kesch C, et al. Multiparametric magnetic resonance imaging (MRI) and MRI-transrectal ultrasound fusion biopsy for index tumor detection: correlation with radical prostatectomy specimen. Eur Urol. 2016; 70:846–53.

6. Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol. 2015; 68:438–50.

7. Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, version 2. Eur Urol. 2016; 69:16–40.

8. Moore CM, Kasivisvanathan V, Eggener S, Emberton M, Futterer JJ, Gill IS, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol. 2013; 64:544–52.

9. Barrett T, Turkbey B, Choyke PL. PI-RADS version 2: what you need to know. Clin Radiol. 2015; 70:1165–76.

10. Futterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, Kirkham A, et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol. 2015; 68:1045–53.
11. Andreoiu M, Cheng L. Multifocal prostate cancer: biologic, prognostic, and therapeutic implications. Hum Pathol. 2010; 41:781–93.

12. Le JD, Tan N, Shkolyar E, Lu DY, Kwan L, Marks LS, et al. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol. 2015; 67:569–76.

13. Wang RS, Kim EH, Vetter JM, Fowler KJ, Shetty AS, Mintz AJ, et al. Determination of the role of negative magnetic resonance imaging of the prostate in clinical practice: is biopsy still necessary? Urology. 2017; 102:190–7.

14. Parry MA, Srivastava S, Ali A, Cannistraci A, Antonello J, Barros-Silva JD, et al. Genomic evaluation of multiparametric magnetic resonance imaging-visible and -nonvisible lesions in clinically localised prostate cancer. Eur Urol Oncol. 2019; 2:1–11.

15. Rouviere O, Puech P, Renard-Penna R, Claudon M, Roy C, Mege-Lechevallier F, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019; 20:100–9.
16. van der Leest M, Cornel E, Israel B, Hendriks R, Padhani AR, Hoogenboom M, et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naive men with elevated prostate-specific antigen: a large prospective multicenter clinical study. Eur Urol. 2019; 75:570–8.
17. Rais-Bahrami S, Siddiqui MM, Vourganti S, Turkbey B, Rastinehad AR, Stamatakis L, et al. Diagnostic value of biparametric magnetic resonance imaging (MRI) as an adjunct to prostate-specific antigen (PSA)-based detection of prostate cancer in men without prior biopsies. BJU Int. 2015; 115:381–8.

18. Porter KK, King A, Galgano SJ, Sherrer RL, Gordetsky JB, RaisBahrami S. Financial implications of biparametric prostate MRI. Prostate Cancer Prostatic Dis. 2020; 23:88–93.

19. Grignon DJ. Prostate cancer reporting and staging: needle biopsy and radical prostatectomy specimens. Mod Pathol. 2018; 31:S96–109.

20. Xiang J, Yan H, Li J, Wang X, Chen H, Zheng X. Transperineal versus transrectal prostate biopsy in the diagnosis of prostate cancer: a systematic review and meta-analysis. World J Surg Oncol. 2019; 17:31.

21. Shoji S. Magnetic resonance imaging-transrectal ultrasound fusion image-guided prostate biopsy: current status of the cancer detection and the prospects of tailor-made medicine of the prostate cancer. Investig Clin Urol. 2019; 60:4–13.

22. Ali A, Hoyle A, Baena E, Clarke NW. Identification and evaluation of clinically significant prostate cancer: a step towards personalized diagnosis. Curr Opin Urol. 2017; 27:217–24.
23. Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015; 313:390–7.

24. Park KJ, Kim MH, Kim JK, Cho KS. Characterization and PI-RADS version 2 assessment of prostate cancers missed by prebiopsy 3-T multiparametric MRI: correlation with whole-mount thin-section histopathology. Clin Imaging. 2019; 55:174–80.

25. Mai Z, Zhou Z, Yan W, Xiao Y, Zhou Y, Liang Z, et al. The transverse and vertical distribution of prostate cancer in biopsy and radical prostatectomy specimens. BMC Cancer. 2018; 18:1205.

26. Priester A, Natarajan S, Khoshnoodi P, Margolis DJ, Raman SS, Reiter RE, et al. Magnetic resonance imaging underestimation of prostate cancer geometry: use of patient specific molds to correlate images with whole mount pathology. J Urol. 2017; 197:320–6.

27. Borghesi M, Ahmed H, Nam R, Schaeffer E, Schiavina R, Taneja S, et al. Complications after systematic, random, and image-guided prostate biopsy. Eur Urol. 2017; 71:353–65.

28. Hu Y, Ahmed HU, Carter T, Arumainayagam N, Lecornet E, Barzell W, et al. A biopsy simulation study to assess the accuracy of several transrectal ultrasonography (TRUS)-biopsy strategies compared with template prostate mapping biopsies in patients who have undergone radical prostatectomy. BJU Int. 2012; 110:812–20.

Fig. 1.
Diagnostic accuracy and value of MRI-US fusion transperineal targeted and systematic biopsy for the detection of clinically significant prostate cancer. bpMRI-US, bi-parametric magnetic resonance imaging–ultrasound; ROI, regions of interest; PI-RADS, Prostate Imaging-Reporting and Data System; FTB, fusion transperineal targeted biopsy; FSB, fusion template systematic biopsy.
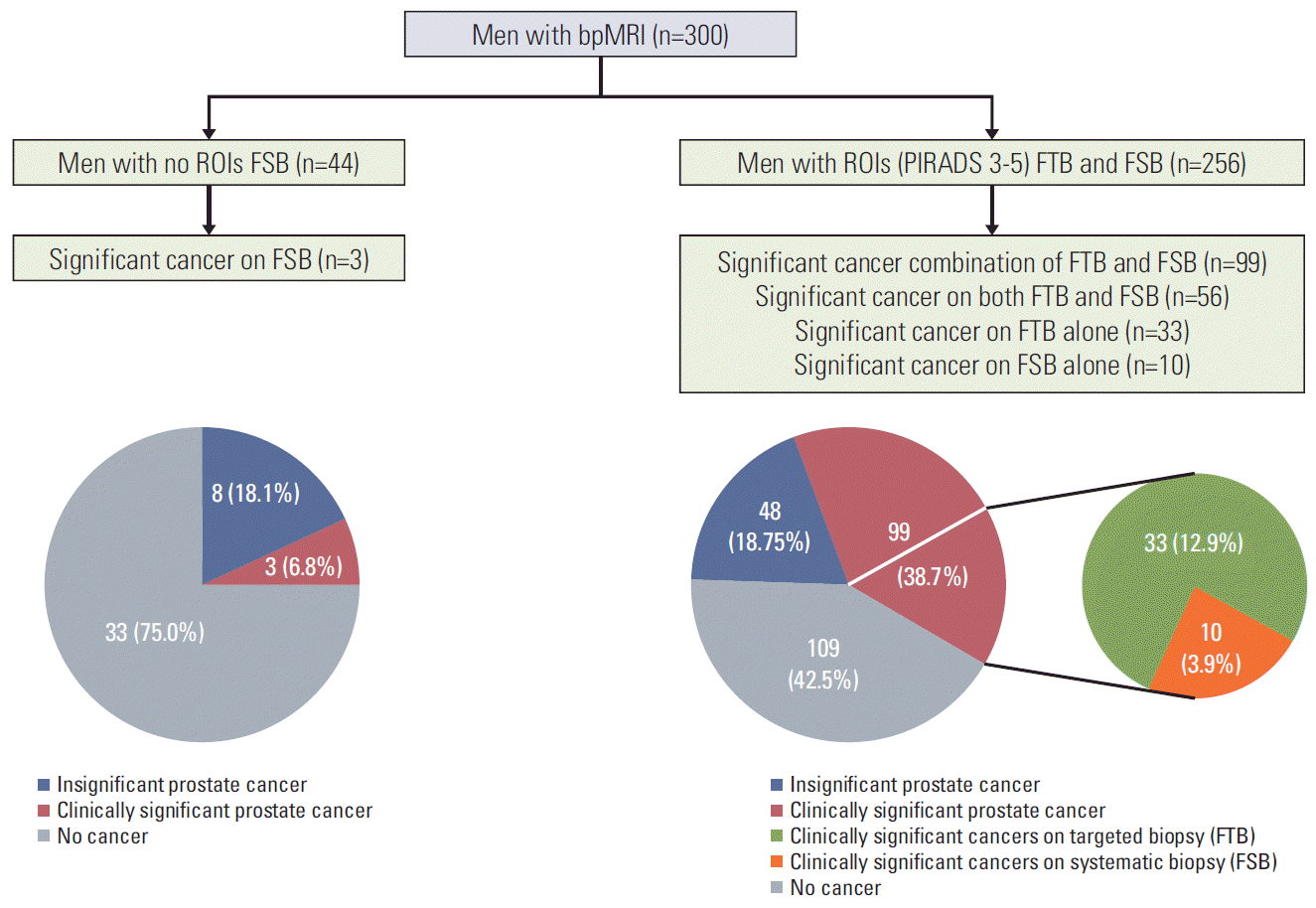
Table 1.
Baseline characteristics of patients
| Characteristic | Total (n=300) | Naive (n=215) | Repeat (n=85) | p-value |
|---|---|---|---|---|
| Age (yr) | 66.6±9.0 | 65.9±9.5 | 68.4±7.6 | 0.017a) |
| BMI (kg/m2) | 24.7±2.5 | 24.9±2.5 | 24.4±2.5 | 0.175a) |
| PSA (ng/mL) | 11.3±21.5 | 11.3±24.3 | 11.4±11.8 | 0.952a) |
| Prostate volume (cm3) | 40.9±19.0 | 39.5±18.7 | 44.6±19.3 | 0.046a) |
| PSA density | 0.33±0.61 | 0.34±0.67 | 0.32±0.41 | 0.735a) |
| DRE nodule | 45 (15.0) | 32 (14.9) | 13 (15.3) | 0.843b) |
| PI-RADS score | 0.429c) | |||
| 0-2 | 44 (14.7) | 36 (16.7) | 8 (9.4) | |
| 3 | 102 (34.0) | 72 (33.5) | 30 (35.3) | |
| 4 | 92 (30.7) | 63 (29.3) | 29 (34.1) | |
| 5 | 62 (20.7) | 44 (20.5) | 18 (21.2) | |
| Fusion targeted biopsy | ||||
| Core | 5.3±3.3 | 5.0±3.2 | 6.2±3.6 | 0.032d) |
| Positive core | 1.7±2.4 | 1.7±2.5 | 1.5±2.3 | 0.570d) |
| Positive core length (mm) | 3.4±4.4 | 3.6±4.5 | 2.9±4.1 | 0.217d) |
| Fusion systematic biopsy | ||||
| Core | 18.6±4.8 | 18.0±4.6 | 19.9±5.1 | 0.004d) |
| Positive core | 2.7±4.0 | 2.7±4.1 | 2.6±3.7 | 0.773d) |
| Positive core length (mm) | 3.0±3.8 | 3.3±4.1 | 1.9±2.5 | 0.277d) |
| Operation (biopsy) time (min) | 29.3±11.6 | 29.5±12.1 | 28.8±10.5 | 0.635a) |
Table 2.
Detection rate of bpMRI-US transperineal FTSB
bpMRI-US, bi-parametric magnetic resonance imaging–ultrasound; FTSB, fusion targeted and systematic biopsy; CI, confidence interval; FTB, fusion transperineal targeted biopsy; FSB, fusion template systematic biopsy; PCa, prostate cancer; PI-RADS, Prostate Imaging-Reporting and Data System; csPCa, clinically significant prostate cancer; GS, Gleason score.
Table 3.
Detection rate of bpMRI-US transperineal FTSB biopsy by history of prior biopsy
Table 4.
Diagnostic accuracy of bpMRI in the detection of PCa and csPCa
Table 5.
The complication rate of bpMRI-US transperineal FTSB based on the Clavien-Dindo system
| Complication | No. (%) |
|---|---|
| Negative | 206 (68.7) |
| Positivea) | 94 (31.3) |
| Grade 1: Hematuria | 67 (22.3) |
| Grade 2: Acute urinary retention | 27 (9.0) |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download