Abstract
Purpose
Little is known about combination of the circulating Epstein-Barr viral (EBV) DNA and tumor volume in prognosis of stage II nasopharyngeal carcinoma (NPC) patients in the intensity modulated radiotherapy (IMRT) era. We conducted this cohort study to evaluate the prognostic values of combining these two factors.
Materials and Methods
By Kaplan-Meier, we compare the differences of survival curves between 385 patients with different EBV DNA or tumor volume levels, or with the combination of two biomarkers mentioned above.
Results
Gross tumor volume of cervical lymph nodes (GTVnd, p < 0.001) and total tumor volume (GTVtotal, p < 0.001) were both closely related to pretreatment EBV DNA, while gross tumor volume of nasopharynx (GTVnx, p=0.047) was weakly related to EBV DNA. EBV DNA was significantly correlated with progress-free survival (PFS, p=0.005), locoregional-free survival (LRFS, p=0.039), and distant metastasis-free survival (DMFS, p=0.017), while GTVtotal, regardless of GTVnx and GTVnd, had a significant correlation with PFS and LRFS. The p-values of GTVtotal for PFS and LRFS were 0.008 and 0.001, respectively. According to GTVtotal and pretreatment EBV DNA level, patients were divided into a low-risk group (EBV DNA 0 copy/mL, GTVtotal < 30 cm3; EBV DNA 0 copy/mL, GTVtotal ≥ 30 cm3; or EBV DNA > 0 copy/mL, GTVtotal < 30 cm3) and a high-risk group (EBV DNA > 0 copy/mL, GTVtotal ≥ 30 cm3). When patients in the low-risk group were compared with those in the high-risk group, 3-year PFS (p=0.003), LRFS (p=0.010), and DMFS (p=0.031) rates were statistically significant.
Nasopharyngeal carcinoma (NPC) is an endemic malignant tumor in South China and South Asia with obvious ethnic aggregation and geographical differences [1-3]. It was once reported that NPC reached a peak incidence of 50 cases per 100,000 individuals [4]. According to the 2016 National Comprehensive Cancer Network (NCCN), radiotherapy alone is a standard treatment modality for patients with stage I NPC due to its anatomic location and relative radiosensitivity. Cisplatin-based concurrent chemoradiotherapy (CCRT) is recommended for patients with stage II NPC. Although our previous study had revealed that CCRT can improve 5-year overall survival (OS), progress-free survival (PFS), and distant metastasis-free survival (DMFS) rates by nearly 10% in the stage II NPC patients, that study was only based on 2-dimensional radiotherapy [5].
Recently, serial studies revealed that intensity modulated radiotherapy (IMRT) improved survival rates compared with 2-dimensional radiotherapy and 3-dimentional radiotherapy and was considered a preferred radiotherapy for NPC patients [6,7]. However, several studies also revealed that CCRT could not improve survival rates of stage II NPC patients, including PFS, locoregional-free survival (LRFS) and DMFS, compared with IMRT [8,9]. Therefore, in the IMRT era, whether CCRT can provide survival benefit for stage II NPC patients treated by IMRT remained in debate. Moreover, currently, there is still a lack of effective biomarkers to select those who have a higher risk of developing distant metastasis and to precisely predict the prognosis for stage II patients. If that come true, the stage II patients with low risk suffering treatment failure may only need to received radiation alone, and the patients with high risk to develop distant metastasis may need more intensive treatment.
In the past 20 years, the survival and local control rate of NPC patients has been greatly improved, and major pattern of treatment failure for stage II NPC patients is still distant metastasis [10]. Currently, the TNM staging system has long been recognized as the most important prognostic indicator for NPC patients, but patients with apparently equivalent International Union against Cancer classification, which is based on nasopharyngeal anatomy, may display a mysterious heterogeneity and receive a totally different outcome [11].
Recently, Mutirangura et al. [12] reported that Epstein-Barr viral (EBV) DNA was closely correlated with long-term survival of NPC patients, and quantification of pretreatment plasma EBV DNA has been widely used as a reliable biomarker for diagnosis, risk stratification, monitoring and prediction of NPC prognosis [13-15]. While tumor volume is an important factor in the prognosis of NPC treatment according to several recent studies [16,17]. One study revealed that the stage II patients with a large primary tumor volume (> 60 mL) had significantly inferior local control and diseasespecific survival, with the 5-year local control rate of 56% and 5-year survival rate of 53% [16]. Given that both pretreatment EBV DNA and tumor volume were independent prognostic factors for advanced NPC patients, whether combining pretreatment EBV DNA and tumor volume will improve the prognostic stratification for stage II patients is still unknown. Therefore, this study was to evaluate whether combining pretreatment plasma EBV DNA levels and tumor volume can refine the prognosis and complement the TNM system to guide individual treatment for stage II patients in the clinical practice.
In all, 385 non-metastatic patients with histologically confirmed primary NPC were prospectively recruited between January 2011 and December 2013. The eligibility criteria are as follows: (1) histologically confirmed primary NPC; (2) no treatment before entering this study; (3) Eastern Cooperative Oncology Group (ECOG) status of 0 to 2; (4) age ≥ 18 years; (5) normal renal and hepatic function; (6) no evidence of distant metastases at initial diagnosis; and (7) complete pretreatment EBV DNA and tumor volume data. Patient groups are as follows: (1) lactation or pregnancy; (2) history of previous or synchronous secondary malignant tumors; (3) refusing treatment or quitting therapy during the process of receiving treatment; or (4) loss of the follow-up data were excluded. All patients were staged as stage II according to the seventh edition of the International Union against Cancer/American Joint Committee on Cancer staging system for NPC.
In our study, 198 patients were treated with IMRT alone, and the remaining 187 patients were treated with CCRT. Details of the radiotherapy techniques were reported in a previous study [18], with the dose of 68-70 Gy for the primary region and 60-68 Gy for metastatic cervical lymph nodes. For the 187 patients treated with CCRT, 142 patients received chemotherapy consisting of cisplatin given on the first, fourth, and seventh week of radiotherapy, and 45 patients received chemotherapy consisting of cisplatin given every week during radiotherapy.
Before treatment, blood samples were taken from each eligible patient for measurement of the plasma EBV DNA level. Blood samples were saved temporarily in EDTA-containing tubes until routinely measured by a real-time quantitative polymerase chain reaction. The detailed method was described in previous studies [19]. In this study, we divided the patients into a detectable group (> 0 copy/mL) and an undetectable group (0 copy/mL) according to the pretreatment plasma EBV DNA level.
At first, all patients received the computed tomography (CT) simulation scan (Plus 4, Siemens, Erlangen, Germany), including plain and enhanced CT, which extended from the top of head to the 2-cm region below the sub-clavicle head with a thickness of 3 mm. Then, contouring of the target region was determined by an IMRT planning system (NOMOS, Pittsburgh, PA) based on the institutional treatment protocol [20]. Our treatment plans were reviewed and approved by at least three radiation oncologists. Finally, tumor volume, regardless of gross tumor volume of nasopharynx (GTVnx) and gross tumor volume of cervical lymph node (GTVnd), was calculated by the IMRT planning system. GTVtotal was calculated by the addition of GTVnx and GTVnd (GTVtotal=GTVnx+GTVnd). In our study, regional lymph nodes with a shortest axial diameter of 11 mm in the jugulodigastric region, 5 mm in the retropharyngeal region and 10 mm in all the other regions of the neck were considered malignant. Besides, if there was a group of three or more lymph nodes with a borderline size, and those with necrosis or extracapsular spread in an irrespective size, these were also considered malignant.
The following baseline clinical data of all eligible patients were collected before treatment: sex, age, treatment, smoking status, concurrent diseases, pathological type, performance status grade evaluated by the ECOG, hereditary NPC, invasion of retropharyngeal lymph nodes, cervical lymph nodes and parapharyngeal space, viral capsid antigen (VCA)‒IgA, early antigen (EA)‒IgA [21,22], plasma EBV DNA level, GTVnx, and GTVnd.
After completion of their treatment, patients were evaluated at 3-month intervals for the first 3 years and then at 6-month intervals in the following years. In this study, our primary endpoint was PFS, OS, LRFS, and DMFS were our secondary endpoints.
PFS was calculated from the date of initial treatment of NPC to the date of first relapse at any site, to the date of death due to any cause, or to the date of the last follow-up visit if patients were still alive. OS was calculated from the date of initial treatment of NPC to the date of death due to any cause, or to the date of the last follow-up visit of the patient. LRFS and DMFS were calculated from the date of initial treatment of NPC to the date of locoregional relapse and distant relapse, respectively, or to the date of the last follow-up visit. Patients who were still alive on the last follow-up date were censored on the date of the last follow-up. Patients who lost contact in the process of following up were censored on the date of the last contact.
In our study, characteristics of the patients were described by the frequency and their corresponding percentages. Continuous variables in this study were described by median values if non-normal or mean (standard deviation), followed by their 25th-75th percentiles (interquartile range [IQR]). We also utilized the receiver operating characteristic (ROC) curve analysis to choose the optimum cutoff point of total tumor volume (GTVtotal),GTVnx, and GTVnd. In brief, the specificity and sensitivity for the outcome being studied at each cutoff point were plotted to generate a ROC curve. The one localized closest to the point at both maximum specificity and sensitivity was chosen to be the optimum cutoff point. The Spearman correlation test was performed to evaluate the relationship between EBV DNA levels and GTVtotal, GTVnx, and GTVnd. The Mann-Whitney U test was utilized to compare the differences of the plasma EBV DNA level and that of GTVtotal in different subgroups separated by the involvement status of retropharyngeal lymph nodes, cervical lymph nodes and parapharyngeal space or clinical outcomes (locoregional failure, distant failure, or death). The Kaplan-Meier method and log-rank test were then used to evaluate the differences in survival probabilities for PFS, OS, LRFS, and DMFS between four subgroups divided according to the EBV DNA levels and tumor volume (details as follows). Potentially important prognostic factors (age, sex, treatment, smoking status, NPC history, involvement of parapharyngeal space, retropharyngeal lymph node and cervical lymph node, VCA-IgA, EA-IgA and combination of tumor volume and EBV DNA) were entered into multivariate analysis using Cox proportional hazards model. Finally, hazard ratios (HRs) as well as their corresponding 95% confidence intervals (CIs) were estimated by means of the Cox proportional hazards regression. All p-values quoted in this study were two-sided, and a p-value of ≤ 0.05 was considered to be statistically significant. Statistics analyses were all performed utilizing the IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY).
The characteristics of the 385 eligible patients with stage II NPC are listed in Table 1. Among these 385 NPC patients, 12 patients finally developed locoregional failure alone, 11 patients developed distant failure alone, one patient developed both locoregional and distant failures, and six patients died due to any cause. The median follow-up time was 49.87 months, with an IQR of 16.22 (42.01-58.23). The 3-year PFS rate, LRFS rate, DMFS rate, and OS rate were 93.5%, 96.6%, 96.9%, and 98.4%, respectively. The median value of the EBV DNA was 0 copy/mL, with a detection rate of 41.8%. The median values of the GTVtotal, GTVnx, and GTVnd were 25.70 cm3, 16.30 cm3, and 6.70 cm3, respectively.
Of the 385 eligible patients, the median EBV DNA levels of the patients with (n=25) or without (n=360) progression were 601.0 copies/mL (IQR, 0 to 16,450.0) and 0 copy/mL (IQR, 0 to 1,687.5), respectively (p=0.008). Median EBV DNA levels of the patients with (n=12) or without (n=373) distant failure were 2,545.0 copies/mL (IQR, 122.3 to 20,875.0) and 0 copy/mL (IQR, 0 to 1,935.0), respectively (p=0.013). For GTVtotal, the median values with (n=25) or without (n=360) progression were 35.20 cm3 (IQR, 22.75 to 50.35) and 24.60 cm3 (IQR, 16.80 to 35.95), respectively (p=0.015). Median values with (n=13) or without (n=372) locoregional failure were 38.50 cm3 (IQR, 31.65 to 55.65) and 24.70 cm3 (IQR, 16.80 to 36.38), respectively (p=0.002).
The detectable rates of pretreatment plasma EBV DNA for patients with or without involvement of retropharyngeal lymph nodes, cervical lymph nodes and parapharyngeal space were 46.8% vs. 32.6%, 54.9% vs. 28.4%, and 46.7% vs. 38.1%, respectively. The median EBV DNA level and GTVtotal of patients with involvement of retropharyngeal lymph nodes were 0 copy/mL (IQR, 0 to 4,105.0) and 27.8 cm3 (IQR, 19.2 to 40.9), respectively, compared with 0 copy/mL (IQR, 0 to 513.0, p=0.003) and 21.6 cm3 (IQR, 15.2 to 32.4, p < 0.001) for patients without involvement of retropharyngeal lymph nodes. Those of patients with involvement of cervical lymph nodes were 345.0 copies/mL (IQR, 0 to 6,710.0) and 30.6 cm3 (IQR, 19.6 to 43.9), compared with 0 copy/mL (IQR, 0 to 94.0, p < 0.001) and 23.1 cm3 (IQR, 15.4 to 31.1; p < 0.001) for patients without metastasis of cervical lymph nodes. GTVtotal for patients with or without involvement of parapharyngeal space was 29.6 cm3 (IQR, 20.6 to 44.0) and 23.2 cm3 (IQR, 15.4 to 34.4; p < 0.001), respectively. Detailed values are listed in Table 2.
As described above, the optimum cutoff point of tumor volume was determined by the ROC curve analysis. As presented in S1A Fig., because our primary endpoint was PFS in this study, the optimum cutoff point of tumor volume for PFS was 29.35 cm3. In addition, for potential acceptance and application in clinical procedures, we chose the nearest integer, 30 cm3, as the optimum cutoff point in this study. Similarly, optimum cutoff points of GTVnx and GTVnd were 20 cm3 and 10 cm3, respectively (S1B and S1C Fig.).
By means of Spearman correlation analysis, we found that both GTVtotal and GTVnd were correlated with EBV DNA, with the correlation coefficients of 0.360 (p < 0.001) and 0.379 (p < 0.001), respectively. However, the GTVnx was weakly correlated with EBV DNA, with the correlation coefficient of 0.101 (p=0.047).
In this study, we found that patients with detectable EBV DNA (> 0 copy/mL) had shorter PFS, LRFS, and DMFS compared with those with undetectable EBV DNA (0 copy/mL). The 3-year PFS, LRFS, and DMFS rates for the detectable and undetectable EBV DNA groups were 89.1% vs. 96.4%, 94.3% vs. 98.2%, and 94.2% vs. 98.6%, with corresponding p-values of 0.005, 0.039, and 0.017, respectively (Fig. 1A, S2A and S2B Fig.). However, there was no difference in 3-year OS rate between detectable and undetectable EBV DNA groups (p=0.769) (S2C Fig.). Similarly, we also noted that patients with a larger GTVtotal (≥ 30 cm3) had shorter PFS and LRFS compared with those patients with a smaller GTVtotal (< 30 cm3). The 3-year PFS and LRFS rates for the larger and smaller GTVtotal groups were 89.2% vs. 96.1% and 92.6% vs. 99.1%, respectively, with the corresponding p-values of 0.008 and 0.001 (Fig. 1B, S3A Fig.). There was also no difference in 3-year DMFS (p=0.431) (S3B Fig.) and OS rate (p=0.620) (S3C Fig.) between the larger and smaller GTVtotal groups. Similar phenomena were observed for GTVnx and GTVnd as that of tumor volume (Fig. 1C and D, S4 and S5 Figs.).
According to the results described as above, both EBV DNA and GTVtotal are effective prognostic factors for PFS, LRFS, and DMFS, but not for OS. Therefore, we divided the entire population into four groups according to the cutoff points of pretreatment EBV DNA level and GTVtotal mentioned above: the undetectable EBV DNA (0 copy/mL) and smaller GTVtotal (< 30 cm3) group (group A), the undetectable EBV DNA and larger GTVtotal (≥ 30 cm3) group (group B), the detectable EBV DNA (> 0 copy/mL) and smaller GTVtotal group (group C), and the detectable EBV DNA and larger GTVtotal group (group D). Interestingly, we found that there was no statistical significance for 3-year PFS and DMFS rates between group A and group B, groups B and C, and groups A and C. Therefore, we further divided the patients into the high-risk group (EBV DNA > 0 copy/mL, GTVtotal ≥ 30 cm3) and low-risk group (EBV DNA 0 copy/mL, GTVtotal < 30 cm3; EBV DNA 0 copy/mL, GTVtotal ≥ 30 cm3; or EBV DNA > 0 copy/mL, GTVtotal < 30 cm3). Differences for PFS, LRFS, and DMFS (Fig. 2) were statistically significant between the low-risk group and the high-risk group. The 3-year PFS, LRFS, and DMFS rates between the low-risk and high-risk groups were 95.5% vs. 86.8% (p=0.003), 97.9% vs. 92.3% (p=0.010), and 97.9% vs. 93.4% (p=0.031), respectively.
In our study, Kaplan-Meier method and log-rank test were used to evaluate the differences between the low-risk group and high-risk group in patients treated with radiotherapy (RT) (n=198) alone and in patients treated with CCRT (n=187). Differences between the low-risk group and highrisk group in patients treated with RT alone were not statistically significant for PFS, with 3-year PFS rates of 95.9% vs. 87.0% (p=0.064). Similarly, differences between the low-risk group and high-risk group in patients treated with CCRT were also not statistically significant for PFS, with 3-year PFS rates of 94.8% vs. 86.7% (p=0.055). Detailed information is shown in Fig. 3.
The results of multivariate analysis are listed in Table 3. In this study, we found that only the combination of tumor volume and pretreatment plasma EBV DNA level (low-risk group vs. high-risk group) reached a p-value of ≤ 0.05, with a corresponding HR value of 2.804 (95% CI, 1.113 to 7.064).
To the best of our knowledge, this is the first study to combine tumor volume and pretreatment plasma EBV DNA level to evaluate the prognosis of stage II NPC patients. In our study, we found that both pretreatment plasma EBV DNA and tumor volume were independent factors for PFS. Pretreatment plasma EBV DNA was found to be more closely correlated with distant metastasis, while tumor volume was found to be more closely correlated with locoregional relapse. The combination of these two factors can improve prognostic stratification for stage II NPC patients.
According to the correlation analysis, we found that both GTVnd and GTVtotal had statistically significant relationships with pretreatment plasma EBV DNA. However, GTVnx was weakly correlated to EBV DNA. We also found that both EBV DNA and GTVtotal were statistically significant between patients with or without involvement of retropharyngeal, cervical lymph nodes and parapharyngeal space, indicating that both EBV DNA and tumor volume are closely related to invasion of retropharyngeal, cervical lymph nodes and parapharyngeal space. These results indicate that both EBV DNA and tumor volume may be correlated to prognosis of stage II NPC patients. A former study considered that plasma EBV DNA may come from necrotic tumor cells and indicate the tumor loading [23]. Thus, our results revealed that EBV DNA may have a close relationship with cervical lymph nodes. This finding is the same as a previous study [24]. It further confirms the result that EBV DNA levels were significantly different in patients with or without distant metastasis, due to the close relationship between distant metastasis and lymph nodes. These results led us to evaluate whether combining the tumor volume and EBV DNA will improve prognostic stratification for stage II NPC patients.
In our study, we also found that patients with detectable pretreatment plasma EBV DNA had a shorter 3-year PFS, LRFS, and DMFS rate compared with those with undetectable EBV DNA. It revealed that EBV DNA is an effective indicator for predicting disease progress or distant relapse. This result is similar to two former studies [24,25]. In addition, we found that patients with larger GTVnx and GTVnd had a shorter 3-year PFS and LRFS rate but not a shorter 3-year DMFS rate. This can be explained because the larger the tumor volume before treatment, the higher the risk of locoregional relapse. These results indicate that both EBV DNA and tumor volume can be effective biomarkers to predict the disease progress for stage II NPC patients. However, the 3-year OS rates were not statistically significant for both EBV DNA and tumor volume. This may be because our follow-up time is not long enough.
When comparing the low-risk group with the high-risk group, we found that 3-year PFS, LRFS, and DMFS rates were all statistically significant. Multivariate analysis showed that only the combination of tumor volume and pretreatment plasma EBV DNA level (low-risk group vs. highrisk group) was significantly correlated with PFS. These results confirm our assumption that the combination of tumor volume and pretreatment plasma EBV DNA level can refine the prognostic value for stage II NPC patients. However, in further analysis, we found that there were no statistically significant differences for PFS between the low-risk group and the high-risk group in patients treated with IMRT alone and those treated with CCRT. This result can also be confirmed by three other studies [13-15]. Moreover, another study showed that there was a statistically significant difference between patients treated with IMRT alone and those treated with CCRT only for LRFS [26]. These results reveal that in the IMRT era, patients with stage II NPC do not gain a better prognosis even when receiving CCRT. A stronger intensity of dose may be necessary for a better prognosis. And it needs our further study to confirm.
To the best of our knowledge, both EBV DNA and tumor volume can be used as biomarkers for predicting the prognosis of NPC, and several studies had confirmed this result. There have been only four studies revealing the relationship between pretreatment plasma EBV DNA and tumor volume [23,27-29]. However, these studies were based on either advanced NPC or all-staged NPC. Neither revealed the relationship between pretreatment plasma EBV DNA and tumor volume in early-staged NPC, especially stage II, which can be treated by radiotherapy alone or CCRT. Therefore, we performed this research based on stage II NPC to evaluate the efficacy of combining pretreatment plasma EBV DNA and tumor volume as a prognostic factor for NPC. This is an advantage of our study.
However, there are also limitations in our research. The first limitation is that our follow-up time is not long enough: there should be further follow-up time if possible. The second limitation is that the measurement of the tumor volume in our study was based on the magnetic resonance imaging and CT scan, since some countries use the positron emission tomography‒computed tomography scans for staging workup (especially in stage III-IV) as the reference for definition of target volumes. Therefore, accurate discrimination of real tumors in different institute could be challenging. The third limitation is that all the eligible patients came from the same center. Therefore, more studies are needed to confirm our study.
In summary, the combination of pretreatment EBV DNA and tumor volume can refine the prognosis of NPC patients and complement the TNM system to reach an excellent prediction. Further studies are needed to set an optimum cutoff point for clinical use. Our study can help indicate individual treatment for NPC patients in future clinical practice.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
ACKNOWLEDGMENTS
We gratefully recognize the patients who participated in this study. This study was partly supported by the Ministry of Science and Technology of China (No. 2011CB504300) and the National Natural Science Foundation of China (81025014, 81230045, 81201629, 91019015, 81071932, 30600755, and 81072226), the 863 Project (No. 2012AA02A501), the National Key Basic Research Program of China (No. 2013CB910304), the Sci-Tech Project Foundation of Guangdong Province (No. 2011B080701034; No. 2011B031800161), the Sci-Tech Project Foundation of Guangzhou City (No. 2011J4300100), the Sun Yat-sen University Clinical Research 5010 Program, the Sun Yat-sen University Cancer Center Clinical Research 308 Program and the Fundamental Research Funds for the Central Universities.
References
1. Choa G. Nasopharyngeal carcinoma. Some observations on the clinical features and technique of examination. Pac Med Surg. 1967; 75:172–4.
2. Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 2011; 30:114–9.

3. Lee AW, Ng WT, Chan YH, Sze H, Chan C, Lam TH. The battle against nasopharyngeal cancer. Radiother Oncol. 2012; 104:272–8.

4. Wee JT, Ha TC, Loong SL, Qian CN. Is nasopharyngeal cancer really a "Cantonese cancer"? Chin J Cancer. 2010; 29:517–26.

5. Chen QY, Wen YF, Guo L, Liu H, Huang PY, Mo HY, et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst. 2011; 103:1761–70.

6. Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011; 80:661–8.
7. Peng G, Wang T, Yang KY, Zhang S, Zhang T, Li Q, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012; 104:286–93.

8. Su Z, Mao YP, Tang J, Lan XW, OuYang PY, Xie FY. Long-term outcomes of concurrent chemoradiotherapy versus radiotherapy alone in stage II nasopharyngeal carcinoma treated with IMRT: a retrospective study. Tumour Biol. 2016; 37:4429–38.

9. Zhang F, Zhang Y, Li WF, Liu X, Guo R, Sun Y, et al. Efficacy of concurrent chemotherapy for intermediate risk NPC in the intensity-modulated radiotherapy era: a propensity-matched analysis. Sci Rep. 2016; 5:17378.

10. Ji MF, Huang QH, Yu X, Liu Z, Li X, Zhang LF, et al. Evaluation of plasma Epstein-Barr virus DNA load to distinguish nasopharyngeal carcinoma patients from healthy high-risk populations in Southern China. Cancer. 2014; 120:1353–60.

12. Mutirangura A, Pornthanakasem W, Theamboonlers A, Sriuranpong V, Lertsanguansinchi P, Yenrudi S, et al. Epstein-Barr viral DNA in serum of patients with nasopharyngeal carcinoma. Clin Cancer Res. 1998; 4:665–9.
13. Chan AT, Lo YM, Zee B, Chan LY, Ma BB, Leung SF, et al. Plasma Epstein-Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst. 2002; 94:1614–9.

14. Lo YM, Chan LY, Lo KW, Leung SF, Zhang J, Chan AT, et al. Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 1999; 59:1188–91.
15. Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS, et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004; 350:2461–70.

16. Chen MK, Chen TH, Liu JP, Chang CC, Chie WC. Better prediction of prognosis for patients with nasopharyngeal carcinoma using primary tumor volume. Cancer. 2004; 100:2160–6.

17. Chua DT, Sham JS, Kwong DL, Tai KS, Wu PM, Lo M, et al. Volumetric analysis of tumor extent in nasopharyngeal carcinoma and correlation with treatment outcome. Int J Radiat Oncol Biol Phys. 1997; 39:711–9.

18. Ma J, Liu L, Tang L, Zong J, Lin A, Lu T, et al. Retropharyngeal lymph node metastasis in nasopharyngeal carcinoma: prognostic value and staging categories. Clin Cancer Res. 2007; 13:1445–52.

19. Tang LQ, Chen QY, Fan W, Liu H, Zhang L, Guo L, et al. Prospective study of tailoring whole-body dual-modality[18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol. 2013; 31:2861–9.
20. Zhao C, Han F, Lu LX, Huang SM, Lin CG, Deng XW, et al. Intensity modulated radiotherapy for local-regional advanced nasopharyngeal carcinoma. Ai Zheng. 2004; 23(11 Suppl):1532–7.
21. Chen WS, Lu JX, Ye SX. Application of combined detection of EBV VCA-IgA and EA-IgA antibodies in the diagnosis of NPC. J Trop Med. 2010; 10:434–6.
22. Cho W. Molecular biomarker discovery and progress in nasopharyngeal carcinoma. Cancer Res. 2007; 67(9 Suppl):Abstr LB-49.
23. Chen M, Yin L, Wu J, Gu JJ, Jiang XS, Wang DJ, et al. Impact of plasma Epstein-Barr virus-DNA and tumor volume on prognosis of locally advanced nasopharyngeal carcinoma. Biomed Res Int. 2015; 2015:617949.

24. Chen WH, Tang LQ, Guo SS, Chen QY, Zhang L, Liu LT, et al. Prognostic value of plasma Epstein-Barr virus DNA for local and regionally advanced nasopharyngeal carcinoma treated with cisplatin-based concurrent chemoradiotherapy in intensity-modulated radiotherapy era. Medicine (Baltimore). 2016; 95:e2642.

25. Lo YM, Leung SF, Chan LY, Chan AT, Lo KW, Johnson PJ, et al. Kinetics of plasma Epstein-Barr virus DNA during radiation therapy for nasopharyngeal carcinoma. Cancer Res. 2000; 60:2351–5.
26. Guo Q, Lu T, Lin S, Zong J, Chen Z, Cui X, et al. Long-term survival of nasopharyngeal carcinoma patients with Stage II in intensity-modulated radiation therapy era. Jpn J Clin Oncol. 2016; 46:241–7.

27. Ma BB, King A, Lo YM, Yau YY, Zee B, Hui EP, et al. Relationship between pretreatment level of plasma Epstein-Barr virus DNA, tumor burden, and metabolic activity in advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006; 66:714–20.

Fig. 1.
Progression-free survival by Kaplan-Meier analysis, comparing the undetectable Epstein-Barr virus (EBV) DNA group with the detectable EBV DNA group (A), larger and smaller gross target volume of nasopharynx (GTVnx)+gross target volume of cervical lymph node (GTVnd) (GTVtotal) group (B), GTVnx group (C), and GTVnd group (D).
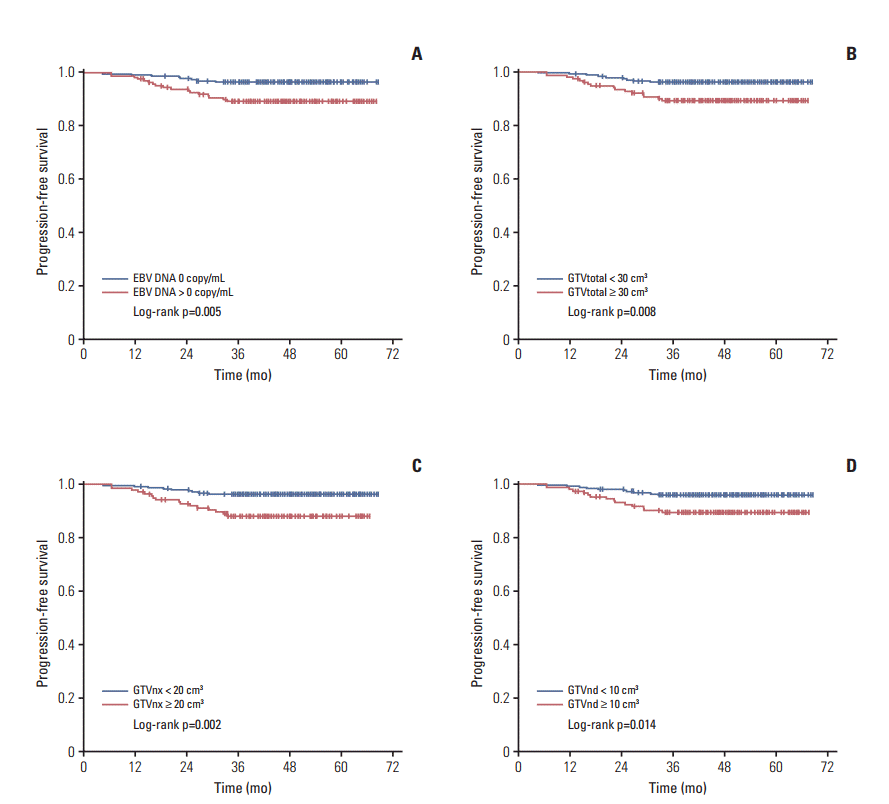
Fig. 2.
Progression-free survival (A), distant metastasis-free survival (B), and locoregional-free survival (C) by Kaplan-Meier analysis, comparing the low-risk group (Epstein-Barr virus [EBV] DNA 0 copy/mL, gross target volume of nasopharynx+ gross target volume of cervical lymph node [GTVtotal] < 30 cm3or ≥ 30 cm3; or EBV DNA > 0 copy/mL, GTVtotal < 30 cm3) and high-risk group (EBV DNA > 0 copy/mL, GTVtotal ≥ 30 cm3).

Fig. 3.
Differences between low-risk group (Epstein-Barr virus [EBV] DNA 0 copy/mL, gross target volume of nasopharynx+gross target volume of cervical lymph node [GTVtotal] < 30 cm3or ≥ 30 cm3; or EBV DNA > 0 copy/mL, GTVtotal < 30 cm3) and high-risk group (EBV DNA > 0 copy/mL, GTVtotal ≥ 30 cm3) in patients treated with intensity modulated radiotherapy (A) and concurrent chemoradiotherapy (B) for progression-free survival by Kaplan-Meier analysis.
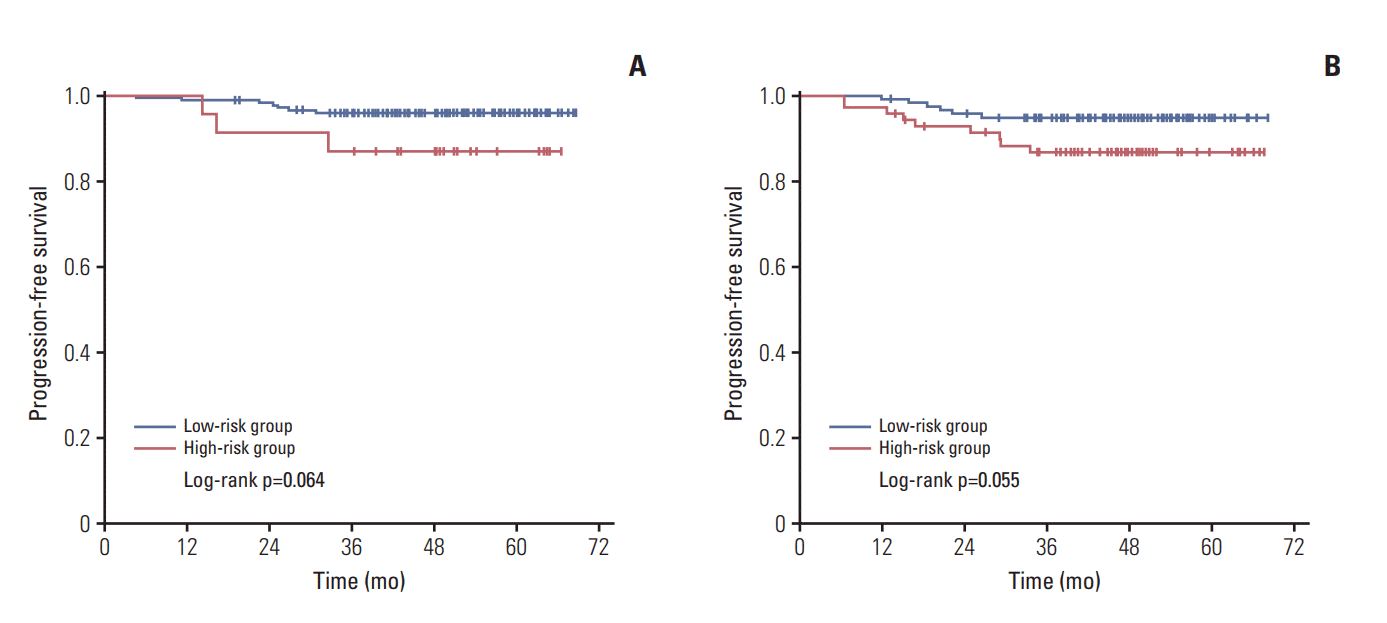
Table 1.
Patient characteristics
IMRT, intensity-modulated radiotherapy; WHO, World Health Organization; ECOG, Eastern Cooperative Oncology Group; NPC, nasopharyngeal carcinoma; VCA, viral capsid antigen; EA, early antigen; EBV, Epstein-Barr virus; GTVtotal, GTVnx+GTVnd; GTVnx, gross target volume of nasopharynx; GTVnd, gross target volume of cervical lymph node.
Table 2.
Differences of EBV DNA and tumor volume in the subgroups with or without specific events
Table 3.
Multivariate Cox proportional hazards analysis




 Citation
Citation Print
Print


 XML Download
XML Download