1. Glezer A, Bronstein MD. Prolactinomas, cabergoline, and pregnancy. Endocrine. 2014; 47:64–9.

2. Molitch ME. Prolactin-secreting tumors: what's new? Expert Rev Anticancer Ther. 2006; 6 Suppl 9:S29–35.

3. Booth AK, Gutierrez-Hartmann A. Signaling pathways regulating pituitary lactotrope homeostasis and tumorigenesis. Adv Exp Med Biol. 2015; 846:37–59.

4. Heaney AP, Fernando M, Melmed S. Functional role of estrogen in pituitary tumor pathogenesis. J Clin Invest. 2002; 109:277–83.

5. Caporali S, Imai M, Altucci L, Cancemi M, Caristi S, Cicatiello L, et al. Distinct signaling pathways mediate stimulation of cell cycle progression and prevention of apoptotic cell death by estrogen in rat pituitary tumor PR1 cells. Mol Biol Cell. 2003; 14:5051–9.

6. Jaffrain-Rea ML, Petrangeli E, Ortolani F, Fraioli B, Lise A, Esposito V, et al. Cellular receptors for sex steroids in human pituitary adenomas. J Endocrinol. 1996; 151:175–84.

7. Colao A, Di Sarno A, Guerra E, De Leo M, Mentone A, Lombardi G. Drug insight: cabergoline and bromocriptine in the treatment of hyperprolactinemia in men and women. Nat Clin Pract Endocrinol Metab. 2006; 2:200–10.

8. Van De Weerdt C, Peers B, Belayew A, Martial JA, Muller M. Far upstream sequences regulate the human prolactin promoter transcription. Neuroendocrinology. 2000; 71:124–37.

9. Oh MC, Aghi MK. Dopamine agonist-resistant prolactinomas. J Neurosurg. 2011; 114:1369–79.

10. Gillam MP, Molitch ME, Lombardi G, Colao A. Advances in the treatment of prolactinomas. Endocr Rev. 2006; 27:485–534.

11. Missale C, Losa M, Boroni F, Giovanelli M, Balsari A, Spano PF. Nerve growth factor and bromocriptine: a sequential therapy for human bromocriptine-resistant prolactinomas. Br J Cancer. 1995; 72:1397–9.

12. Gao H, Wang F, Lan X, Li C, Feng J, Bai J, et al. Lower PRDM2 expression is associated with dopamine-agonist resistance and tumor recurrence in prolactinomas. BMC Cancer. 2015; 15:272.

13. Cai L, Leng ZG, Guo YH, Lin SJ, Wu ZR, Su ZP, et al. Dopamine agonist resistance-related endocan promotes angiogenesis and cells viability of prolactinomas. Endocrine. 2016; 52:641–51.

14. Galluzzi L, Morselli E, Kepp O, Tajeddine N, Kroemer G. Targeting p53 to mitochondria for cancer therapy. Cell Cycle. 2008; 7:1949–55.

15. Tokino T, Nakamura Y. The role of p53-target genes in human cancer. Crit Rev Oncol Hematol. 2000; 33:1–6.

16. Thapar K, Scheithauer BW, Kovacs K, Pernicone PJ, Laws ER Jr. p53 expression in pituitary adenomas and carcinomas: correlation with invasiveness and tumor growth fractions. Neurosurgery. 1996; 38:765–70.

17. Sav A, Rotondo F, Syro LV, Scheithauer BW, Kovacs K. Biomarkers of pituitary neoplasms. Anticancer Res. 2012; 32:4639–54.
18. Wang Z, Fukushima H, Inuzuka H, Wan L, Liu P, Gao D, et al. Skp2 is a promising therapeutic target in breast cancer. Front Oncol. 2012; 1:18702.

19. Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008; 8:438–49.
20. Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG Jr. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004; 428:194–8.

21. Kitagawa M, Lee SH, McCormick F. Skp2 suppresses p53-dependent apoptosis by inhibiting p300. Mol Cell. 2008; 29:217–31.

22. Lee SH, McCormick F. Downregulation of Skp2 and p27/Kip1 synergistically induces apoptosis in T98G glioblastoma cells. J Mol Med (Berl). 2005; 83:296–307.

23. Amaral JD, Xavier JM, Steer CJ, Rodrigues CM. The role of p53 in apoptosis. Discov Med. 2010; 9:145–52.
24. Shalini S, Dorstyn L, Dawar S, Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015; 22:526–39.

25. Kanasaki H, Fukunaga K, Takahashi K, Miyazaki K, Miyamoto E. Involvement of p38 mitogen-activated protein kinase activation in bromocriptine-induced apoptosis in rat pituitary GH3 cells. Biol Reprod. 2000; 62:1486–94.
26. Deshaies RJ. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu Rev Cell Dev Biol. 1999; 15:435–67.

27. Chan CH, Morrow JK, Li CF, Gao Y, Jin G, Moten A, et al. Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell. 2013; 154:556–68.

28. Chao W, Xuexin Z, Jun S, Ming C, Hua J, Li G, et al. Effects of resveratrol on cell growth and prolactin synthesis in GH3 cells. Exp Ther Med. 2014; 7:923–8.

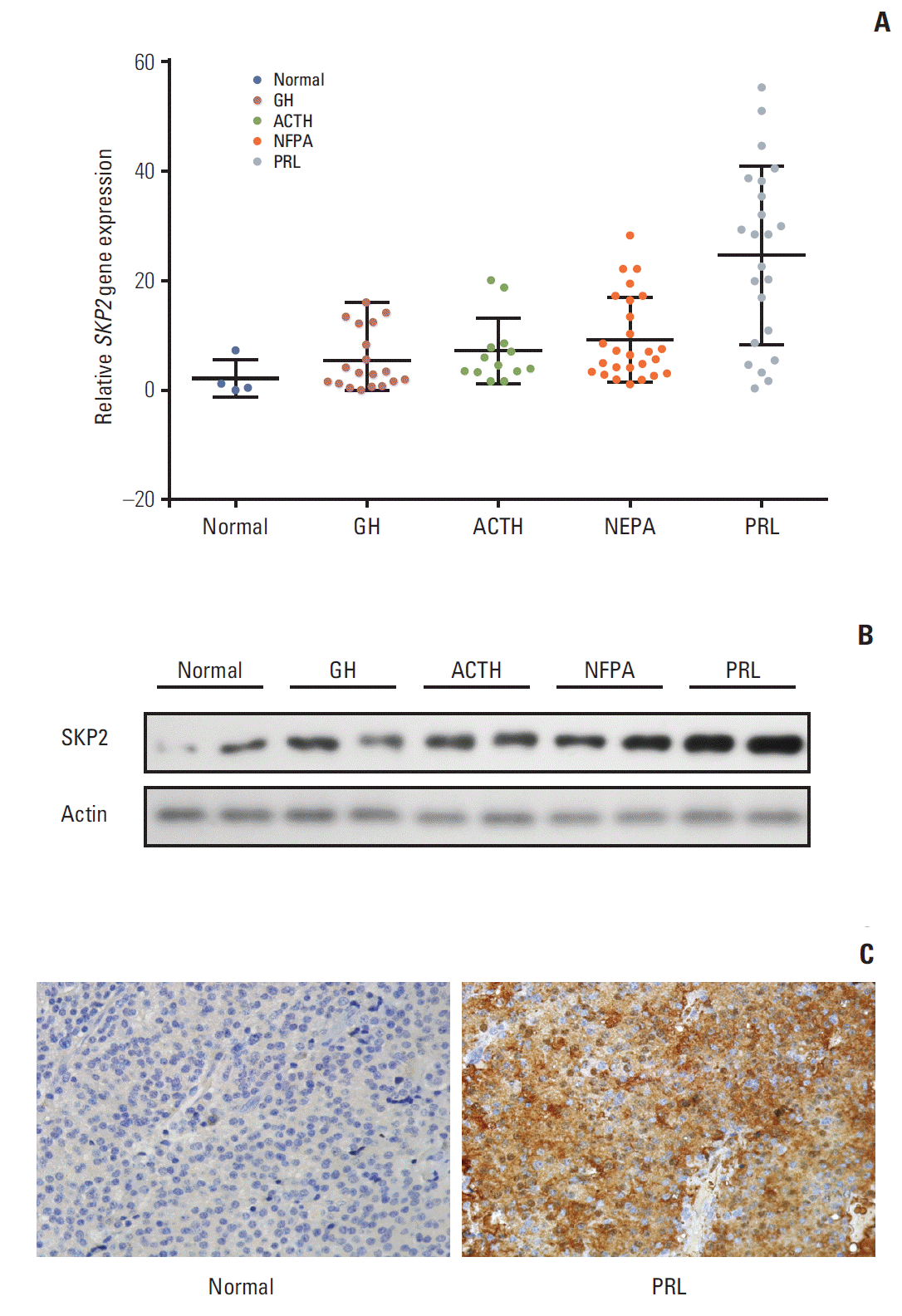
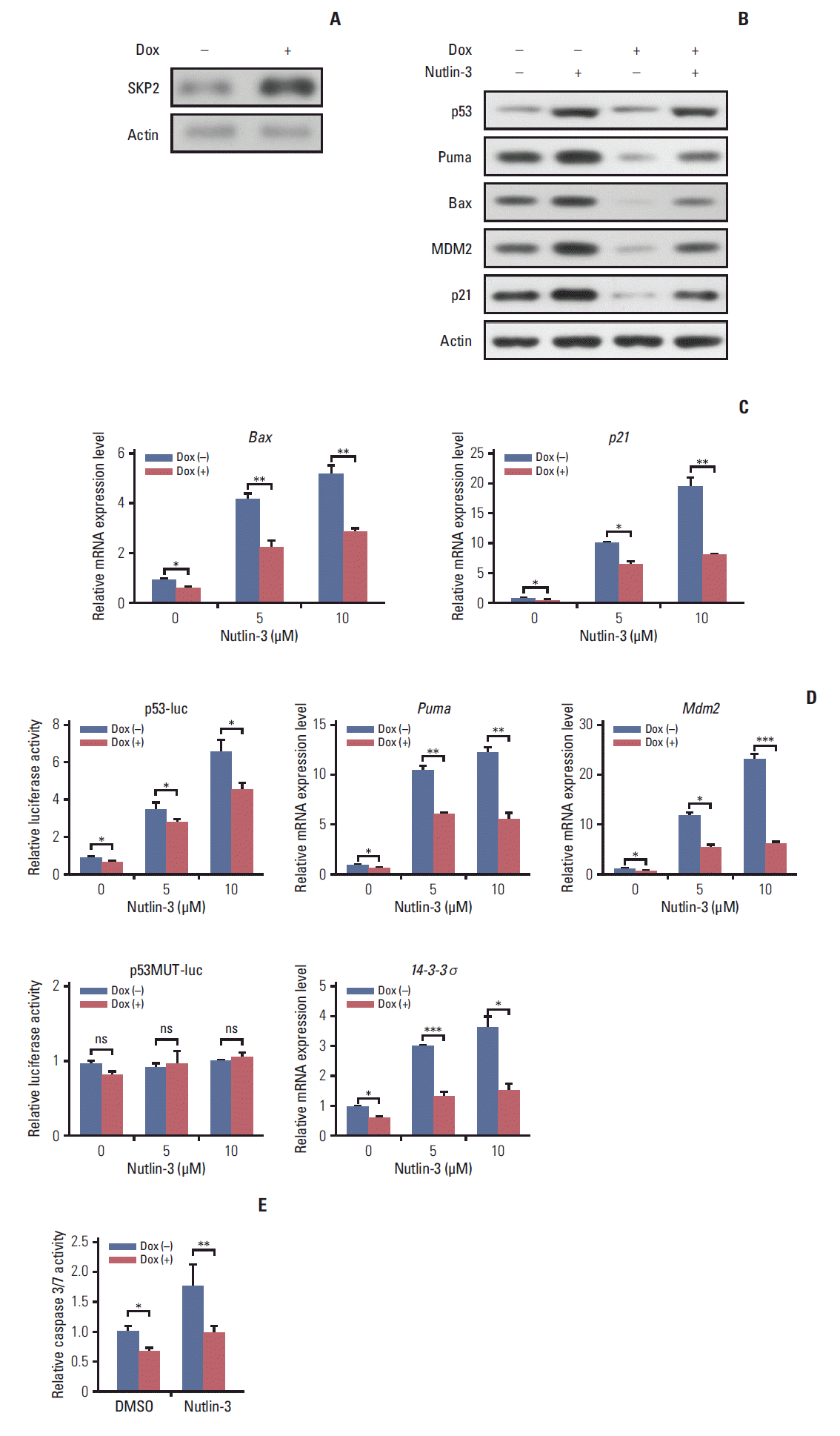
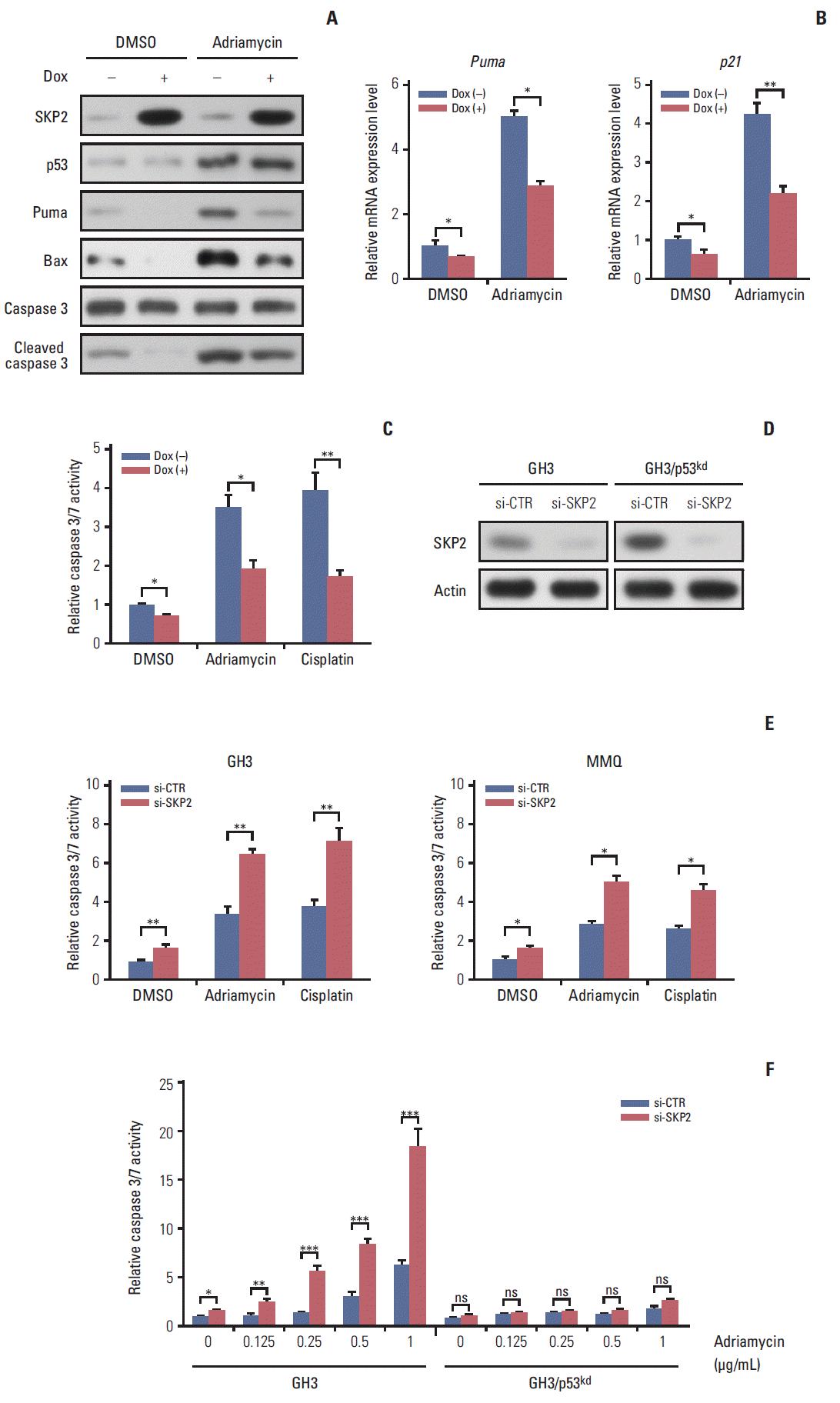
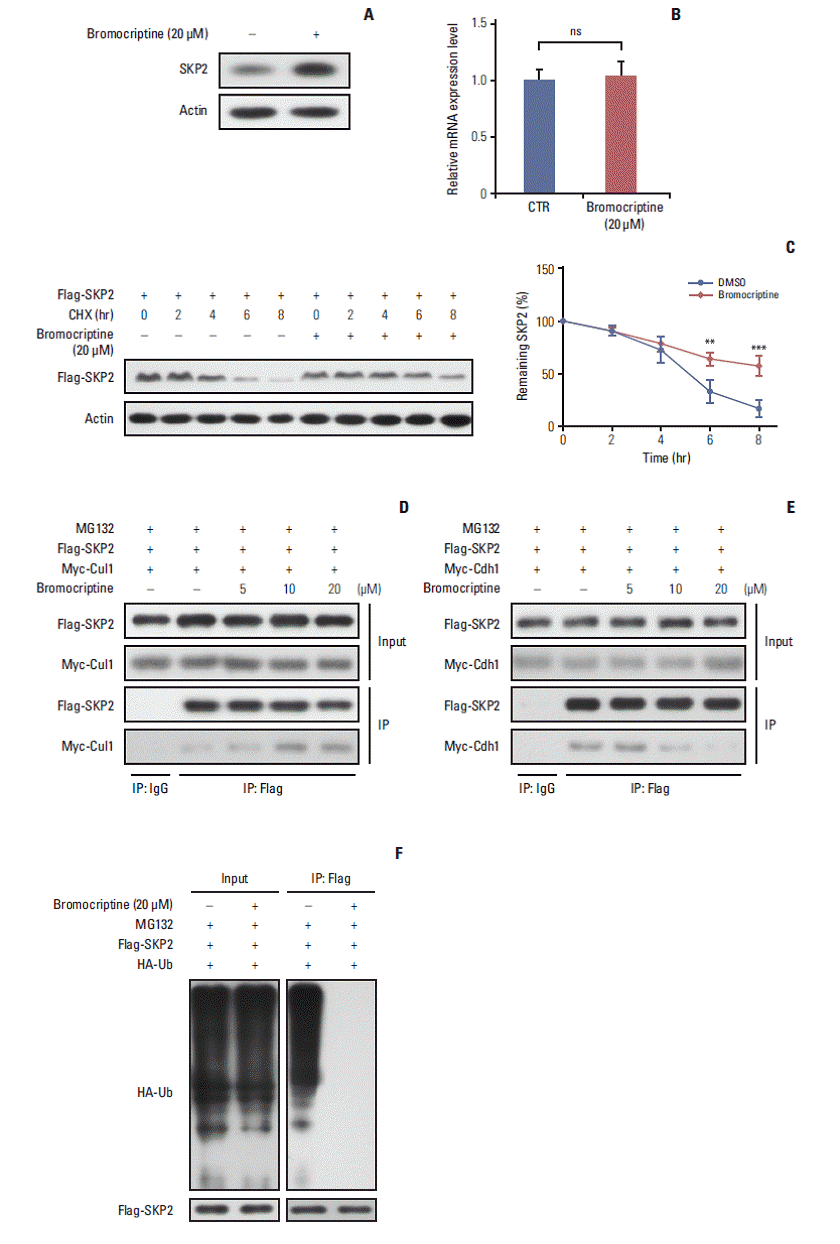
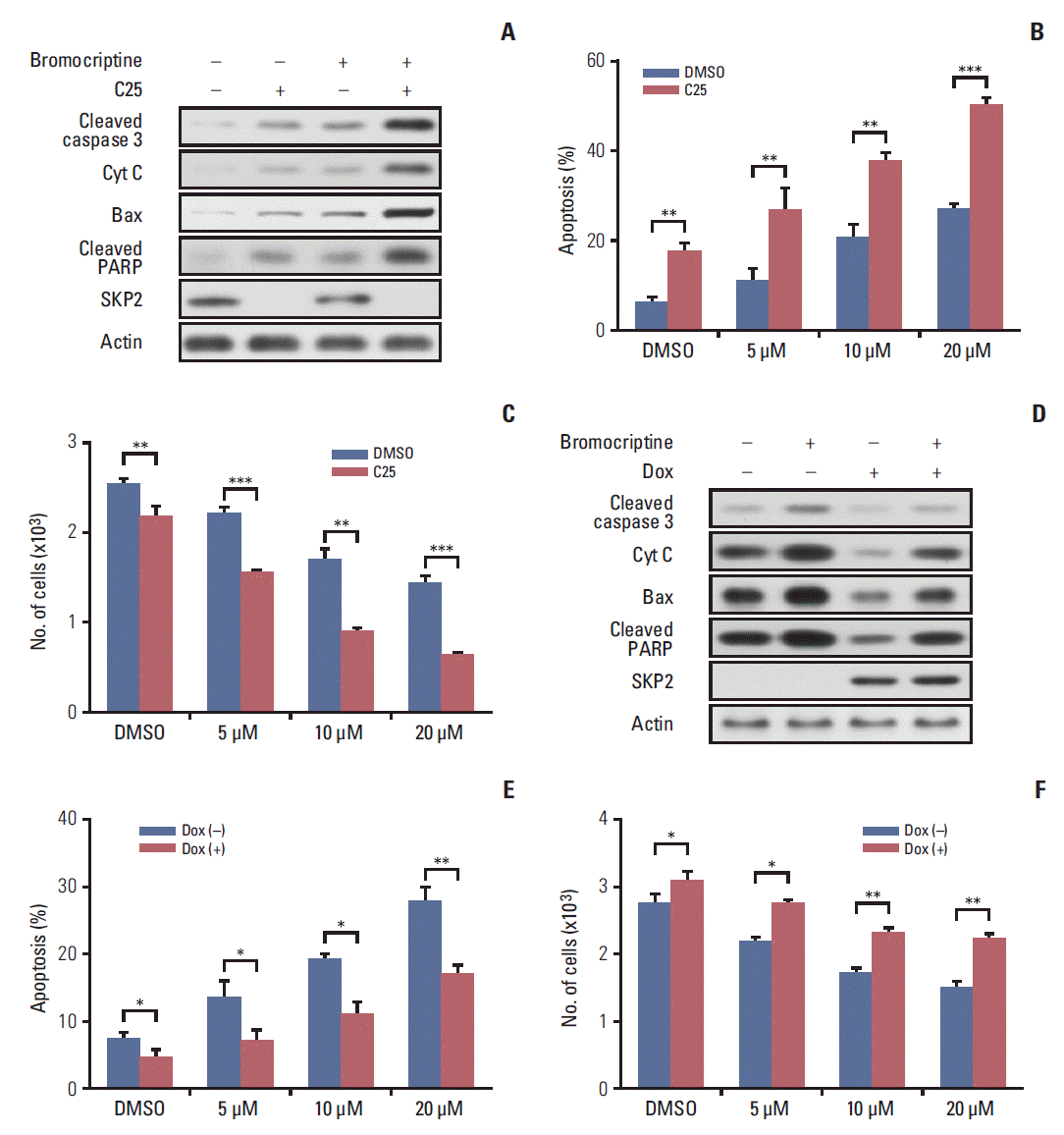
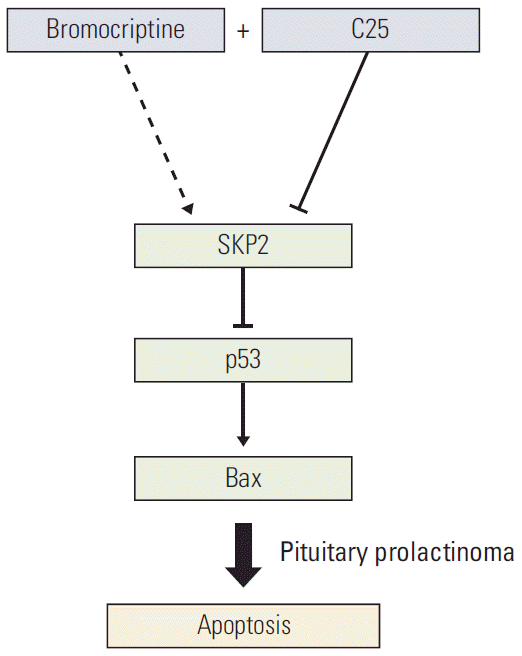




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download