INTRODUCTION
MATERIALS AND METHODS
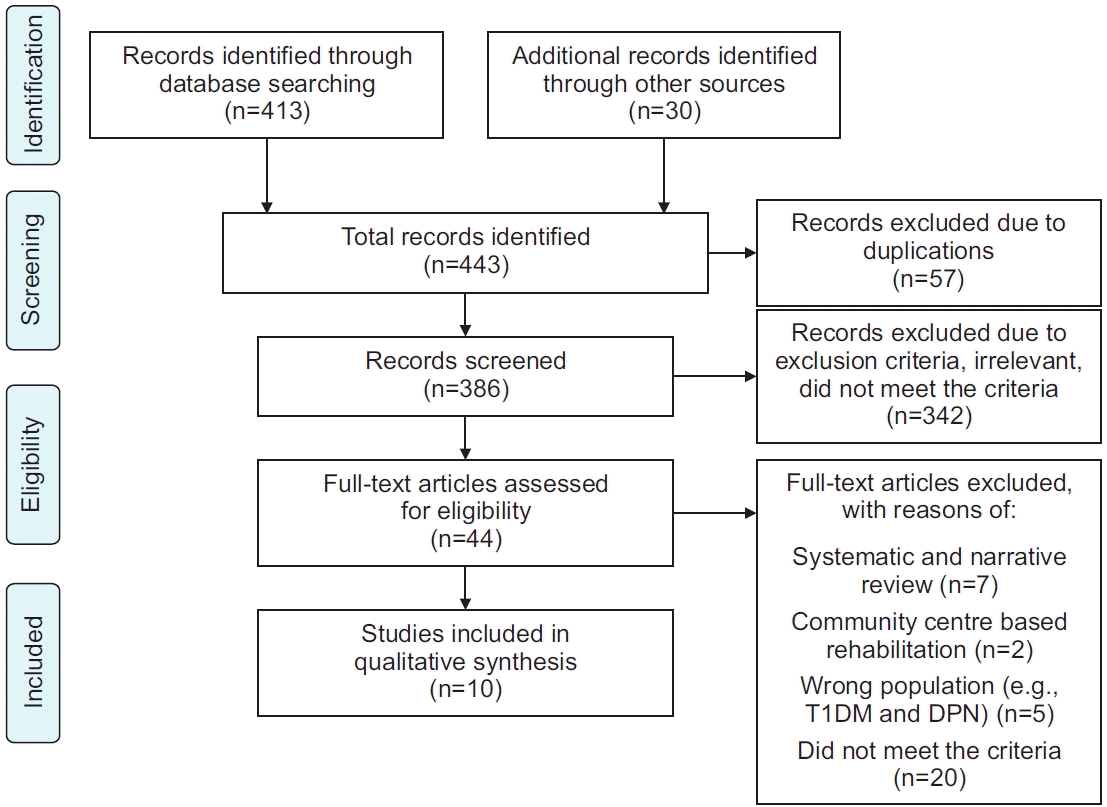
Study design
Search strategy
Eligibility criteria
Study selection
Data extraction
Table 1.
| Study, year | Study design |
Participant characteristics |
Study period (mo) | Exercise intervention |
Exercise protocol |
Supervision/contact | Exercise aids | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Sample size | Mean age (yr) | Sex (M/F) | Freq . session per week | Intensity/volume | Time per session (min)a) | Type/mode | ||||||
| Aylin et al. [20], 2009 | PS | NA | HB: 18 | 51.39±2.02 | 15/3 | 2 | Supervised RE and HB AE | 2 | 50%-60% 1RM | 35 | RE: Shoulder flexion (sandbag); biceps curl, triceps kickback (dumbbell); hip flexion and abduction, knee extension and flexion (ankle weight); sit up | Supervised RE | NA |
| 8-10 reps/2 sets | |||||||||||||
| CG: 18 (no exercise) | 56.06±1.48 | 12/6 | 2 | 60-79% HR max | 10 → 35 | AE: Walking | Weekly phone call | Log quest and feedback | |||||
| IM: HR | |||||||||||||
| Cheung et al. [25], 2009 | Pilot RCT | Sedentary | HB: 20 | 59±8.7 | 7/13 | 4 | HB RE | 5 | 12 reps/2 sets | 30 | Chest press, back rows, leg press, triceps, and biceps curls (dynabands); leg abductions and extension (body weight) | Supervised (5 session in 2 week of 1st m → monthly visit) | Diary |
| CG: 17 (no exercise) | 62±6.7 | 5/12 | |||||||||||
| Dadgostar et al. [26], 2016 | RCT | NA | HB: 36 | 50.21±5.47 | F | 3 | Supervised RE and HB AE | 3 | Low (red) → high intensity (blue) | NA | RE: Shoulder girdle, biceps, triceps, chest, abdomen, back, quadriceps, hamstring and calf muscles (theraband) | Supervised RE (3 session/week in 1st 6 week → 1 session/week in 2nd 6 week | Pedometer and activity log |
| 10-15 reps/2-3 sets | |||||||||||||
| CG: 36 (no exercise) | 49.06±5.81 | 3-5 | 2,500-3,000 → 10,000-12,000 steps/day) | NA | AE: Walking | ||||||||
| IM: talk test | |||||||||||||
| Dunstan et al. [21], 2005 | RCT | Sedentary, elderly, and OW | HB: 16 | 67.6±5.2 | 10/6 | 12 | 1st phase: Supervised RE | 3 | 50%-60% 1RM → 75%-85% 1RM | 45 | Supervised RE: Bench press, leg extension, upright row, lateral pulldown, leg curl (ankle weights); shoulder press, biceps curl, triceps kickback (dumbbell); abdominal curls | Supervised RE | NA |
| 2nd phase: HB RE | 8-10 reps/3 sets | ||||||||||||
| CG: 13 | 66.9±5.3 | 6/7 | 3 | 60%-80% 1RM | NA | HB RE: Lying dumbbell flies; single-leg extension (ankle weights); shoulder press, bent-over row, upright row, bicep curls, triceps kickbacks (dumbbell); leg curl (ankle weights); abdominal curls | Home visit in 1st week | Diary, exercise booklet, log quest and feedback | |||||
| 8-10 reps/3 sets | Weekly phone call in 1st m → 1 phone call in 2 week | ||||||||||||
| Monthly hospital visit | |||||||||||||
| Ferrer-Garcia et al. [22], 2011 | RCT | Elderly | HB: 44 | 65.59±7.6 | 18/26 | 6 | HB RE and AE | 2 | 75%-95% 1RM | NA | RE: Different circuit of big muscle groups | 3 clinic visits in baseline, 3rd m, and 6th m | NA |
| 3 × phone call | |||||||||||||
| 2 visits for diet and exercise compliance | |||||||||||||
| CG: 40 (exercise education) | 67.86±8.44 | 16/24 | 1 | NA | NA | AE: Orienteering hikes, dancing, games, etc. | |||||||
| Krousel-Wood et al. [27], 2008 | FS | Sedentary | HB: 37 | 56.6±9.6 | 24/52 | 3 | HB RE and AE | 5 | Low→moderate | 10 → 30 | RE and AE: Exercise similar to ADL (e.g. walking) | Weekly emailed activity log | Videotape, activity log, and pedometer |
| CG: 39 (no exercise) | |||||||||||||
| Liu et al. [28], 2015 | RCT | NA | HB: 22 | 52.6±11.43 | 12/10 | 3 | HB RE and AE | 3 | 40% VO2 max → 60% VO2 max | 10 → 35 | AE: NA | Supervised AE (3-10 sessions in the beginning) | NA |
| IM: Borg scale | Monthly hospital visit | ||||||||||||
| Weekly phone call | |||||||||||||
| CG: 20 (no exercise) | 51.2±11.34 | 7/13 | 2-3 | 50%-60% 1RM | NA | RE: Using elastic band | |||||||
| 8-10 reps/2 sets | |||||||||||||
| IM: Borg scale | |||||||||||||
| Plotnikoff et al. [29], 2010 | RCT | Sedentary and obese | HB: 27 | 55±12 | 8/9 | 4 | HB RE | 3 | 50%-60% 1RM → 85% 1RM | NA | RE: Core: squats, seated row, chest press, shoulder press; complementary: lunges, lateral pull-down, triceps extension, pulley abdominal twists, biceps curl, triceps press, reverse rhomboid flies, lateral pulley deltoid raise and pulley abdominal curls | Home supervised 3 sessions in 1st 2 week → 2 sessions in 2nd 2 week → weekly in 2nd m → 1 session in 2 week of 3rd m | Activity log |
| 8-10 reps/2-3 sets | |||||||||||||
| CG: 21 (no exercise) | 54±12 | 8/13 | |||||||||||
| Rekha et al. [30], 2014 | RCT | NA | HB: 28 | 43.38±5.74 | 13/15 | 1 | HB AE | 5 | 12-14 Borg scale | 30 | AE: Brisk walking | Weekly hospital visit | Activity log |
| CG: 25 (no exercise) | 44±6.36 | 14/11 | |||||||||||
| Shinji et al. [31], 2007 | PS | NA | HB: 64 | 58.3±9.8 | 40/24 | Avg. 17.2 | HB AE | 4-6 | Anaerobic threshold | 20-30 | AE: Daily walking | Phone call | NA |
Values are presented as mean±standard deviation.
ADL, activities of daily living; AE, aerobic exercise; CG, control group; FS, feasibility study; HB, home-based; HR, heart rate; IM, intensity monitoring; NA, not available; OW, overweight; PS, prospective study; Quest, questions; Reps, repetitions; RE, resistance exercise; RM, repetitive maximum; →, progression to.
Table 2.
| Study, year | Exercise intervention |
Outcome measures |
Main findings | Adherence/dropouts (%/%) | Significant adverse events | ||||
|---|---|---|---|---|---|---|---|---|---|
| Glycemic control | Body composition | Physical performance | QoL | Others | |||||
| Aylin et al. [20], 2009 | Combined | HbA1c | BMI | RM | SF-36 | Lipid | HbA1c and muscle strength improveda) | 96/5 | No |
| FBG | FBG, QoL, and lipid improvedb) | ||||||||
| No improvement in BMI | |||||||||
| Cheung et al. [25], 2009 | RE | HbA1c | BMI | Handgrip | SF-36 | PA (AAQ) | HbA1c, body composition, physical performance, and level of PA improvedb) | 90/7.5 | Not reported |
| Waist and hip C | TUG | ||||||||
| CSR | QoL improvedb) except general health itema) | ||||||||
| SLB | |||||||||
| Dadgostar et al. [26], 2016 | Combined | HbA1c | BMI | NA | SF-36 | Lipid | BMI, body fat, lipid improveda) | 77.8/27 | Not reported |
| FBG | Body fat | PA (step count) | HbA1c, FBG, waist circumference, gluteal girth, and level of PA improvedb) | ||||||
| Waist C | QoL improvedb) except general health and physical itema) | ||||||||
| Gluteal girth | |||||||||
| Dunstan et al. [21], 2005 | RE | HbA1c | Weight | RM | NA | IS | FBG, muscle strength, IS, and level of PA improvedb) | 88/19 | No |
| FBG | Body fat | Habitual PA | Muscle strength improvedc) | ||||||
| LBM | HbA1c, and body composition improvedd) | ||||||||
| Waist C | |||||||||
| Ferrer-Garcia et al. [22], 2011 | Combined | HbA1c | BMI | NA | HRQoL | Lipid | HbA1c, FBG, and QoL improveda) | 63/12 | No |
| BBG | Waist C | BMI, waist circumference, and lipid improvedb) | |||||||
| Krousel-Wood et al. [27], 2008 | Combined | HbA1c | BMI | NA | PF-10 | BP | HbA1c, BMI, QoL, and BP improvedb) | 80% in 12 participants/19 | No |
| Liu et al. [28], 2015 | Combined | HbAlc | NA | NA | NA | Lipid | HbA1c, PBG, lipid, IR, and inflammatory markers improveda) | Not reported/0 | Not reported |
| PBG | IR | ||||||||
| Inflammatory markers | |||||||||
| Plotnikoff et al. [29], 2010 | RE | HbAlc | BMI | RM | SF-12 | Lipid | HbA1c, FBG, BMI, waist and hip circumference, QoL, |lipid, improvedb) | 71/15 | No |
| FBG | Waist and hip C | Insulin | |||||||
| SE | Muscle strength and SE improveda) | ||||||||
| Rekha et al. [30], 2014 | AE | FBG | NA | NA | DSQoL | NA | FBG and QoL improveda) | Not reported/3.8 | Not reported |
| Shinji et al. [31], 2007 | AE | NA | NA | NA | NA | CVD | Adherence to exercise is associated with a reduced incidence of CVD | Attended 4 sessionse)/37.3 | Not reported |
AAQ, Active Australia Questionnaire; AE, aerobic exercise; BMI, body mass index; BP, blood pressure; Combined, combined resistance and aerobic exercises; C, circumference; CSR, Chair Sit and Reach; CVD, cardiovascular disease; DSQoL, diabetic specific quality of life questionnaire; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; HRQOL, health-related quality of life; IR, insulin resistance; IS, insulin sensitivity; LBM, lean body mass; NA, not assessed; PA, physical activity, PBG, postprandial blood glucose; PF-10, 10-item Physical Functioning Scale; QoL, quality of life; RM, repetitive maximum; SE, self-efficacy; SF-12, Short Form 12; SF-36, Short Form 36; SLB, single leg balance; TUG, Timed Up and Go.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download