INTRODUCTION
Visual function is considered to be one of the most important perceptions for development. During motor development, vision provides crucial feedback to the vestibular and proprioceptive systems [
1]. Vision leads to the development of integrative functions such as eye-hand coordination, visual-manual-oral coordination, object recognition and learning, and visual-spatial recognition and learning. Several studies [
234] on children who were diagnosed as blind or with visual impairment by an ophthalmologist have suggested that children with visual impairment show developmental delay and demonstrated the correlation between visual impairment and development. Also, the reported studies diagnosed the children's vision disorders based on ophthalmic examination, without the use of other vision-testing tools.
There exist few tools to evaluate the visual status of young children. Visual evoked potentials (VEPs) can provide diagnostic information on the functional integrity of the visual system. VEPs are visually evoked electrophysiological signals extracted from the electroencephalographic activity in the visual cortex recorded from the overlying scalp [
5]. VEP testing is a simple, non-invasive, and easily accessible tool for evaluating the visual status of young children who might be uncooperative. Among several types of VEPs, flash VEP, which is less dependent on fixation, is commonly used in young children.
One study that used VEP in preterm infants within 2 weeks of birth identified a good predictive value for the occurrence of cerebral palsy [
6]. However, the diagnosis of cerebral palsy was made at the corrected ages of 12 months in the study, which is too early for definitive diagnosis of cerebral palsy. Furthermore, no other developmental disabilities were assessed except for cerebral palsy. Thus, further studies involving both preterm and full-term babies and a longer follow-up period are required to clarify the diagnosis. In addition, studies using reliable assessment tools to evaluate development status such as the Bayley Scales of Infant and Toddler Development second edition (BSID-II) would reveal detailed information.
Until now, only a few studies have correlated VEP results in children with developmental delay. To our knowledge, there was no previous study which used VEP together with the BSID-II. The BSID-II is a tool used worldwide to evaluate the development of variable cognitive functions as well as the motor performances of infants and children [
7]. Furthermore, the clinical significance of the VEP in children with developmental disorders has not been demonstrated. Therefore, the aim of this study was to investigate the neurodevelopmental outcome of children assessed with the BSID-II according to the VEP results.
Go to :

DISCUSSION
Our current study findings indicate that children with delayed VEP latency results exhibited impediment in both mental and psychomotor development compared with children with normal VEP latency. These findings are consistent with the common concept that vision plays an important role in development and learning and that visual impairment would lead to disturbance in development.
Both mental and psychomotor developments are visually driven. Rolling over, raising up on upper limbs, sitting, crawling, and walking are all believed to be driven initially by visual stimulation triggered by some factor in the environment, coupled with the drive to obtain objects, explore, and manipulate them and thus learn about them. Therefore, a great extent of developmental delay observed in both mental and psychomotor development in the delayed VEP latency group compared to the normal group seems to be reasonable.
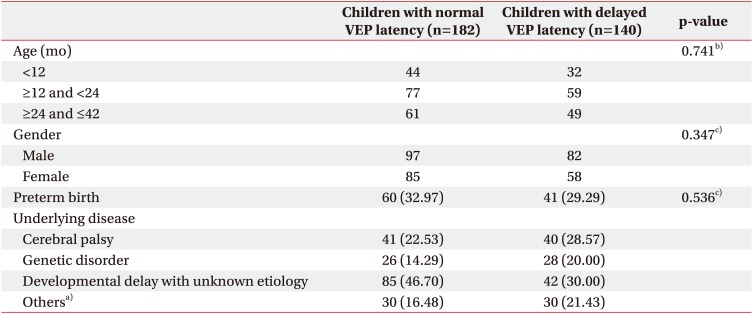
Significant differences were observed in the index scores of BSID between the normal and delayed VEP latency groups including all ages. However, a comparison of the index scores of the subdivided age groups revealed only a significant difference in children aged between 12 and 23 months and not in children younger than12 months and from 24 months to 42 months, possibly because many children had index scores under 50 (assigned a score of 49) in both the groups. Comparisons of the DQs of BSID in the subdivided age groups revealed no significant differences in children younger than 12 months and those aged between 12 and 24 months.
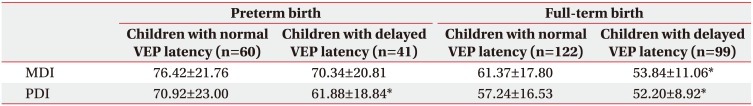
Subgroup analysis based on the birth of children as full-term or preterm revealed no significant difference in MDI between the children born at preterm in both the groups. However, both MDI and PDI showed a significant delay in the delayed VEP latency group in children born at full-term. These results suggest the reliability of VEP in children born at full-term. The previous study on VEP between premature and full-term children demonstrated the vulnerability of VEP abnormality in premature infants than full-term infants [
12].
The results of our present study are consistent with those of the previous reports that stated a correlation between visual impairment and developmental delay. Levtzion-Korach et al. [
2] compared the motor development in blind children with a sighted control group based on Bayley and Denver's motor milestones, and reported a highly significant developmental delay in all the motor skills in the blind children. However, the study only examined the motor development, and children with a mild visual impairment who could see the objects were excluded. A study by Vaizey et al. [
3] reported a correlation between the severity of visual impairment and the incidence of neurodevelopmental delay in a series of 30 patients with congenital retinal dystrophies. However, the case series did not present any developmental assembly and some patients were found to be mentally subnormal. In addition, the participants were limited to Leber congenital amaurosis patients, diagnosed with electroretinography.
Cass et al. [
4] revealed that developmental setback is a significant clinical problem among children with severe visual impairment and is most prevalent in those with the greatest degree of visual impairment. This finding is different from our present analyses as it focused on the developmental setback in children who are initially thought to be undergoing normal development by 16 months of age. Additionally, the participants in the study of Cass et al. [
4] were all diagnosed with visual impairment by an ophthalmologist and the study only focused on cognitive progress and used Reynell-Zinkin scales of mental development, which were designed for young visually handicapped children.
Several studies have investigated the prognostic value of VEP in high-risk newborns. Kato and Watanabe [
13] reviewed the relevant reports and concluded that VEP demonstrates a good correlation with neurodevelopmental outcome in full-term infants with birth asphyxia. However, based on the five articles they reviewed, the prognostic value of VEP in preterm infants was controversial [
6141516]. The five reviewed studies mainly focused on the prevalence of cerebral palsy as a neurodevelopmental outcome. The VEP examinations were performed in the preterm infants within 3 weeks of birth. And the follow-up time of assessing neurodevelopmental status ranged from 12 to 24 months, which is too early to diagnose cerebral palsy. Except for a study that included 123 preterm infants, the others included less than 100 participants.
Our current study reviewed a fairly large number of children and included both full-term and preterm infants. Furthermore, this is the first study to use the BSID-II, a widely accepted reliable developmental assessment tool for evaluating development status, along with VEP. We not only examined the prevalence of the neurodevelopmental disorder but investigated both mental and psychomotor development.
There are several limitations in our study. First, in this retrospective analysis, we could only obtain limited information recorded in the medical chart. Secondly, although most of the children we analyzed underwent the VEP study at around 1 year of age, the time of the VEP study varied from several months to 3 years. In addition, though the P100 latency of VEP changes rapidly in form and complexity in the first 6 months and reaches the typical adult value by 1 year of age [
1117], the latency in preterm infants can be a little more delayed by 5 months [
18]. Although the VEP study is typically done after 6 months of age in our hospital, we should be cautious with the interpretation of this value.
Though in the present study, as per the result of VEP normal and delayed latency groups were designed, the value of the latency was not checked. Therefore we could not concretely suggest the degree of the latency delay and further analyze the correlation with delayed development. These aspects need to be considered in the future study. Furthermore, the mean time difference between the VEP study and the BSID test was about 4 months, which is not a short-term for young children. Also, we could not control the extent of rehabilitation undertaken by the participants. It is hypothesized that differences in rehabilitation program intensity would probably affect children's development. Lastly, ophthalmologic interventions, such as prescription of corrective lenses or surgery, were not considered.
In conclusion, children with VEP-proven visual impairment showed a more developmental delay in both mental and psychomotor domains. Although VEP provides limited information, it does have any clinical usefulness. When a physician has concerns about visual impairment, VEP studies could be easily applied to children with suspected developmental delay. In addition, VEP study results could provide an insight into children's development and serve as early indicators for consultation with an ophthalmologist for the existing problem.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download