1. Belagaje SR. Stroke rehabilitation. Continuum (Minneap Minn). 2017; 23(1, Cerebrovascular Disease):238–253. PMID:
28157752.

2. Kwakkel G, van Peppen R, Wagenaar RC, Wood Dauphinee S, Richards C, Ashburn A, et al. Effects of augmented exercise therapy time after stroke: a meta-analysis. Stroke. 2004; 35:2529–2539. PMID:
15472114.
3. Teasell RW, Kalra L. What's new in stroke rehabilitation. Stroke. 2004; 35:383–385. PMID:
14757887.

4. Bhogal SK, Teasell R, Speechley M. Intensity of aphasia therapy, impact on recovery. Stroke. 2003; 34:987–993.

5. Astrand A, Saxin C, Sjoholm A, Skarin M, Linden T, Stoker A, et al. Poststroke physical activity levels no higher in rehabilitation than in the acute hospital. J Stroke Cerebrovasc Dis. 2016; 25:938–945. PMID:
26851969.
6. Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark P, et al. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: a scientific statement from the American Heart Association. Stroke. 2010; 41:2402–2448. PMID:
20813995.
7. Vloothuis JD, Mulder M, Veerbeek JM, Konijnenbelt M, Visser-Meily JM, Ket JC, et al. Caregiver-mediated exercises for improving outcomes after stroke. Cochrane Database Syst Rev. 2016; 12:CD011058. PMID:
28002636.

8. Galvin R, Stokes E, Cusack T. Family-Mediated Exercises (FAME): an exploration of participant's involvement in a novel form of exercise delivery after stroke. Top Stroke Rehabil. 2014; 21:63–74. PMID:
24521841.

9. Gandolfi M, Geroin C, Tomelleri C, Maddalena I, Kirilova Dimitrova E, Picelli A, et al. Feasibility and safety of early lower limb robot-assisted training in sub-acute stroke patients: a pilot study. Eur J Phys Rehabil Med. 2017; 53:870–882. PMID:
28084064.

10. Tsunoda T, Maeshima S, Watanabe M, Nagai A, Ueno Y, Ozeki Y, et al. Rehabilitation for a patient with hemiplegia, ataxia, and cognitive dysfunction caused by pontine hemorrhage. Case Rep Neurol. 2015; 7:213–220. PMID:
26600785.

11. Redzuan NS, Engkasan JP, Mazlan M, Freddy Abdullah SJ. Effectiveness of a video-based therapy program at home after acute stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2012; 93:2177–2183. PMID:
22789773.

12. van den Berg M, Crotty M Prof, Liu E, Killington M, Kwakkel G, van Wegen E. Early supported discharge by caregiver-mediated exercises and e-health support after stroke: a proof-of-concept trial. Stroke. 2016; 47:1885–1892. PMID:
27301941.
13. Galvin R, Cusack T, O'Grady E, Murphy TB, Stokes E. Family-mediated exercise intervention (FAME): evaluation of a novel form of exercise delivery after stroke. Stroke. 2011; 42:681–686. PMID:
21233462.
14. Kalra L, Evans A, Perez I, Melbourn A, Patel A, Knapp M, et al. Training carers of stroke patients: randomised controlled trial. BMJ. 2004; 328:1099. PMID:
15130977.

15. Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One. 2014; 9:e87987. PMID:
24505342.

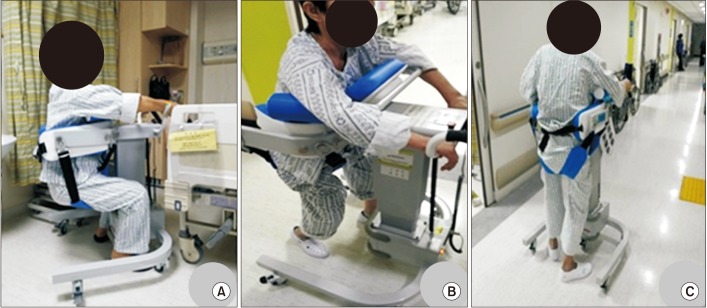
16. Dean CM, Channon EF, Hall JM. Sitting training early after stroke improves sitting ability and quality and carries over to standing up but not to walking: a randomised trial. Aust J Physiother. 2007; 53:97–102. PMID:
17535145.
17. Braun T, Marks D, Thiel C, Zietz D, Zutter D, Gruneberg C. Effects of additional, dynamic supported standing practice on functional recovery in patients with sub-acute stroke: a randomized pilot and feasibility trial. Clin Rehabil. 2016; 30:374–382. PMID:
25952591.

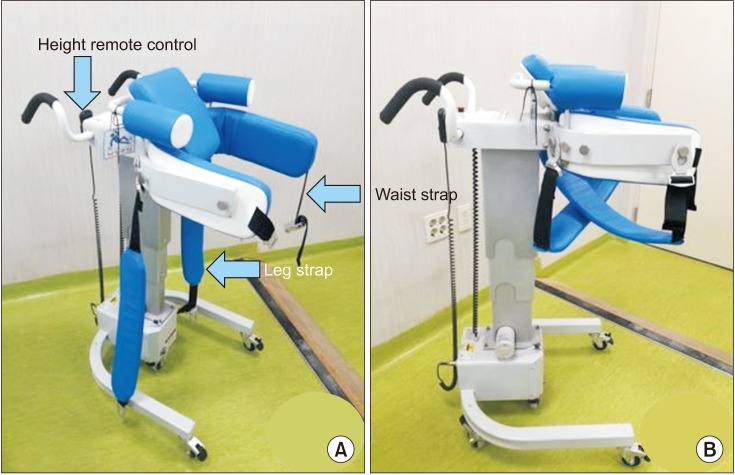
18. Hesse S. Treadmill training with partial body weight support after stroke: a review. NeuroRehabilitation. 2008; 23:55–65. PMID:
18356589.

19. Combs-Miller SA, Kalpathi Parameswaran A, Colburn D, Ertel T, Harmeyer A, Tucker L, et al. Body weight-supported treadmill training vs. overground walking training for persons with chronic stroke: a pilot randomized controlled trial. Clin Rehabil. 2014; 28:873–884. PMID:
24519922.

20. Manning CD, Pomeroy VM. Effectiveness of treadmill retraining on gait of hemiparetic stroke patients. Physiotherapy. 2003; 89:337–349.

21. Hesse S, Uhlenbrock D, Sarkodie-Gyan T. Gait pattern of severely disabled hemiparetic subjects on a new controlled gait trainer as compared to assisted treadmill walking with partial body weight support. Clin Rehabil. 1999; 13:401–410. PMID:
10498347.

22. Gama GL, Celestino ML, Barela JA, Forrester L, Whitall J, Barela AM. Effects of gait training with body weight support on a treadmill versus overground in individuals with stroke. Arch Phys Med Rehabil. 2017; 98:738–745. PMID:
28034719.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download