Abstract
Purpose
Breast cancer diagnosis and treatment often produce stress in patients. Anxiety is one of the most prevalent psychological symptoms perceived by breast cancer patients. This study aims to evaluate the temporal patterns of anxiety and find factors associated with persistent anxiety during breast cancer treatment.
Methods
This is prospective cohort study. Between July 2010 and July 2011, we recruited patients with nonmetastatic breast cancer who were expected to receive adjuvant chemotherapy (n = 411) from 2 cancer hospitals in Seoul, Korea. Anxiety was measured using the Hospital Anxiety and Depression Scale.
Results
The mean age of the participants was 46.4 ± 7.9 years. Preoperatively, 44.5% (183 of 411) of the patients showed abnormal anxiety. The proportion of the abnormal anxiety group significantly decreased after surgery (P < 0.01) and this phenomenon continued until the 12-month follow-up point. Patients experienced renewed anxiety at 12 months when the main adjuvant therapies were finished. Socioeconomic factors were not associated with persistent anxiety. Pain, breast, and arm symptoms were significantly higher in the persistently abnormal group, especially at postoperative months 6 and 12.
Conclusion
Surgery was a major relieving factor of anxiety, and patients who finished their main adjuvant treatment experienced renewed anxiety. Surgeons should be the main detectors and care-givers with respect to psychological distress in breast cancer patients. To reduce persistent anxiety, caring for the patient's physical symptoms is important.
Treatments for breast cancer are long and complicated processes, including surgery, chemotherapy, radiotherapy, endocrine therapy, and target therapy. During the long period of treatment, breast cancer patients experience several diverse psychological distresses, such as depression, anxiety, pain, and social isolation. This psychological distress is a normal and adaptive reaction to unpleasant stimuli or threats. However, it sometimes persists in some patients to where it may damage the individual's quality of life (QoL) [12], adherence to treatment [34], and even clinical outcomes [567] in breast cancer. Therefore, the American College of Surgeons Commission on Cancer and National Comprehensive Cancer Network recommended that cancer patients be routinely screened for psychological distress during the cancer trajectory.
Among psychological distresses, anxiety is very common and has been associated with cancer [1289101112]. However, most studies examining psychological distress have been focused on depression, and the concerns about anxiety have been relatively small compared to depression.
We conducted this prospective longitudinal cohort study to determine the QoL and psychological distress of breast cancer patients who were newly diagnosed with their respective diseases. In this report, we evaluated the temporal patterns of anxiety in patients who underwent breast cancer operations using the Hospital Anxiety Depression Scale (HADS), and found factors associated with persistent anxiety during breast cancer treatment. The HADS [13] is a standardized 14-item self-assessment instrument for the evaluation of anxiety and depression in patients with nonpsychiatric and physical illness (Supplementary Table 1). It has been frequently used in cancer research as a validated, brief and effective screening tool [14]. This was used in this study because it can be easily assessed without proficiency and the Korean versions of the HADS has been validated [15].
This was a prospective longitudinal cohort study that followed consecutive newly diagnosed breast cancer patients 18 years and older from 2 different cancer hospitals in Seoul, Korea, between July 2010 and July 2011. Patients were asked to fill out preoperative questionnaires, and again at 2 weeks, 3 months, 6 months, and 12 months after surgery by themselves. If they had difficulties understanding or filling out the questionnaires, a nurse or trained research assistant helped them to complete the questionnaire.
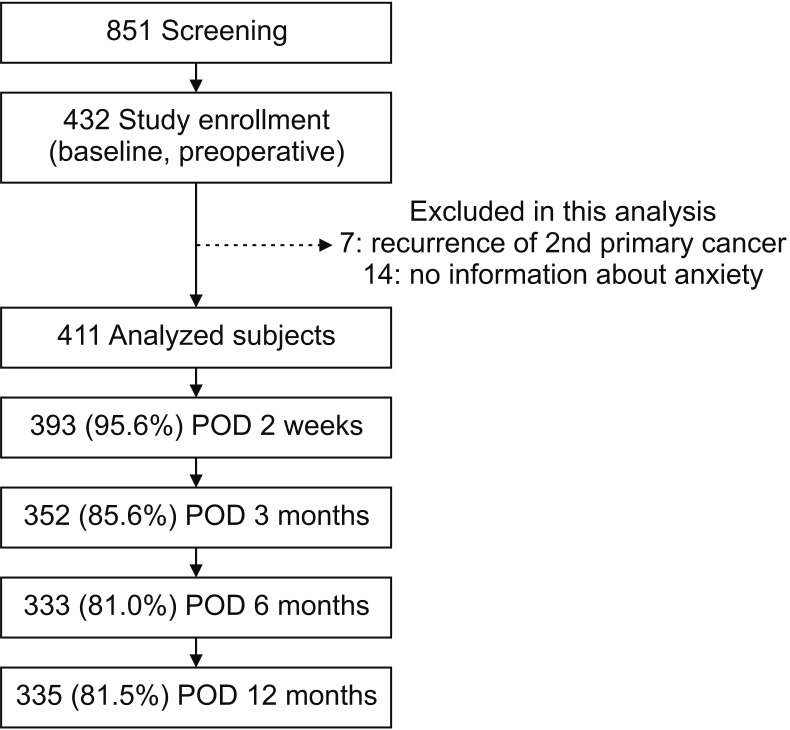
We included patients who underwent breast cancer surgery with pathologic stage 0 to III in this study (n = 851). We excluded patients who received neo-adjuvant treatment and had secondary cancer, or who did not understand the purpose of the study or had psychotic disorders at the beginning of the survey. Ultimately, 432 patients (50.8%) agreed to participate in the study. For the analysis of this study for anxiety disorder, 7 patients who had recurrence or a second primary cancer during follow-up and 14 patients who did not have the information about anxiety were excluded. The final analysis included 411 patients (Fig. 1). This study was approved by the Institutional Review Boards (IRB No. SMC 2010-05-069), and all study participants provided written informed consent.
Anxiety was assessed using the patient's completed HADS, which is frequently used as a screening tool for psychological distress in cancer patients. A study was conducted to standardize the HADS for Koreans by Oh et al. [15], which demonstrated the validity and accessibility of the HADS, and we used this Korean version of the questionnaire for this study (Supplementary Table 2). There are 7 questions for the anxiety dimension in HADS (HADS-A) and each item is scored on a scale of 0 to 3, with a higher score indicating a more severe symptom. The scores can be categorized as; 0–7, normal; 8–10, borderline; and 11–21, abnormal. In this analysis, we considered cutoff scores of ≥8 as cases of anxiety including borderline and abnormal level based on the previous report [14].
We used a combined form of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 and the breast cancer specific module (BR-23), which has been translated into Korean and validated [1617]. Symptoms (breast and arm) were measured using the 7 questions of the BR-23. The BR-23 has 23 items to assess the impact of common breast cancer treatment modalities (surgery, chemotherapy, radiotherapy, or hormonal treatment). It includes questions about body image, sexual functioning, sexual enjoyment, and future perspective for function and questions about systemic therapy side effects, breast symptoms, arm symptoms, and distress over hair loss (Supplementary Tables 3, 4). We used additional measured questions related to sociodemographic and clinical characteristics, including age, marital status, income, education, employment status, religion, stage, breast surgery type, and treatment received.
Differences in baseline demographical and clinical characteristics were compared by the severity of the anxiety group using chi-square tests for categorical variables and t-tests for continuous variables. Multilevel models were used to assess how the severity of anxiety affected the level of change in the symptom level over time and case-wise deletion was used for the missing data. This method is functionally the same as growth curve modeling or mixed-effects models. The models were adjusted for baseline demographical characteristics and clinical characteristics, as shown in Table 1. Full-information maximum likelihood estimation was used to directly compare the fit of the nested models to the data and to use all variable data, given the presence of missing data at random. All analyses were conducted using STATA 12.0 (StataCorp LP, College Station, TX). P < 0.05 was considered statistically significant.
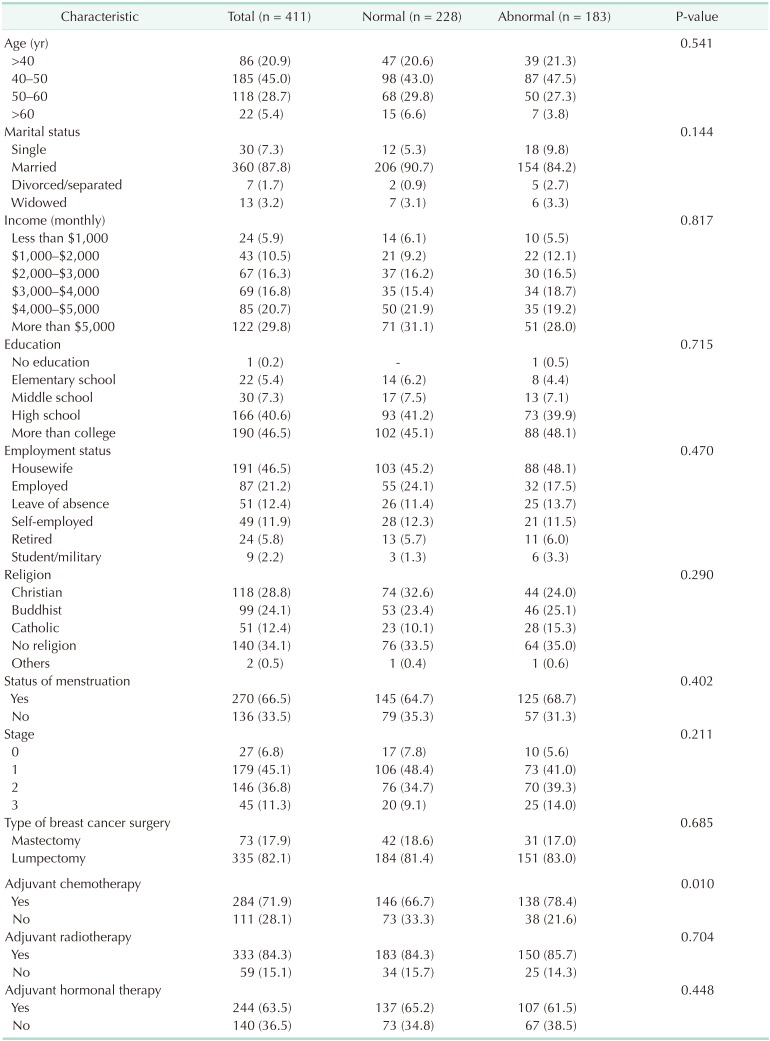
The characteristics of the 411 participants are listed in Table 1. A total of 183 patients (44.5%) showed abnormal levels of anxiety after diagnosis. The patients were regrouped into normal and abnormal groups based on the preoperative level of anxiety. We analyzed socioeconomic factors which could affect the baseline of anxiety; however, nothing was related with baseline anxiety. Age, marital status, income, statuses of education and employment, religion, and menstruation status were not associated with preoperative anxiety. Among clinical factors such as TNM stage and adjuvant therapy, nothing except adjuvant chemotherapy was related with baseline anxiety.
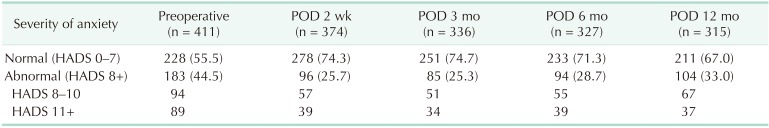
Among 44.5% (183 of 411) of the patients who showed abnormal levels of anxiety preoperatively, 89 patients (48.6%) showed a high score of anxiety (more than 11). After surgery, about half of the patients who had an abnormal level of anxiety preoperatively returned to normal (from 44.5% to 25.7%) (Table 2). We could see the highest level of anxiety at diagnosis with improvement after surgery. As shown in Fig. 2, mean HADS-A score decreased significantly from 7.5 to 5.7 immediately after surgery. The HADS-A score was sustained during the follow-up period; though slightly elevated again at 12 months after the surgery when main adjuvant treatments were completed.
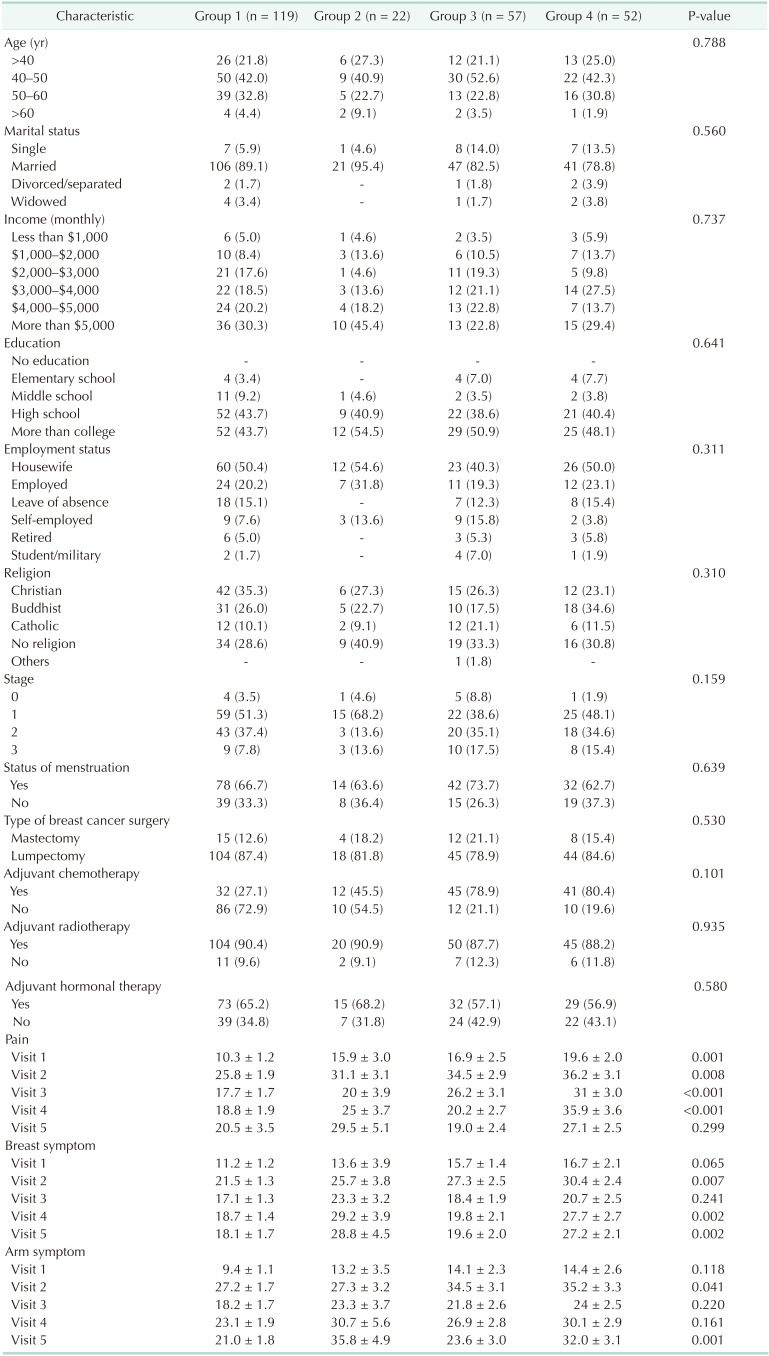
A total of 250 patients with complete longitudinal data about anxiety were analyzed during the follow-up period. We classified these patients into 4 groups: Group 1 (normal), group 2 (newly developed anxiety), group 3 (resolved anxiety), and group 4 (persistent anxiety). Among the 141 patients who showed normal anxiety levels preoperatively, 22 (15.6%) developed anxiety symptoms late, at 12 months (group 2). Fifty-seven patients (52.3%) who showed abnormal levels of anxiety at baseline were normalized 12 months after surgery (group 3). Nonetheless, 29.6% (74 of 250) were still suffering from persistent (group 4) or newly developed (group 2) anxiety (Fig. 3). Fig. 4 shows the temporal pattern of anxiety of each group over time. According to the level of baseline anxiety, overall mean scores and pattern of change were different from each other.
Socioeconomic and clinicopathologic demographics were not associated with persistent anxiety in our data (Table 3). Pain, breast, and arm symptoms were significantly higher in the newly developed or persistent anxiety groups (groups 2 and 4), especially at the time of visits 4 (postoperative day [POD] 6 months) and 5 (POD 12 months).
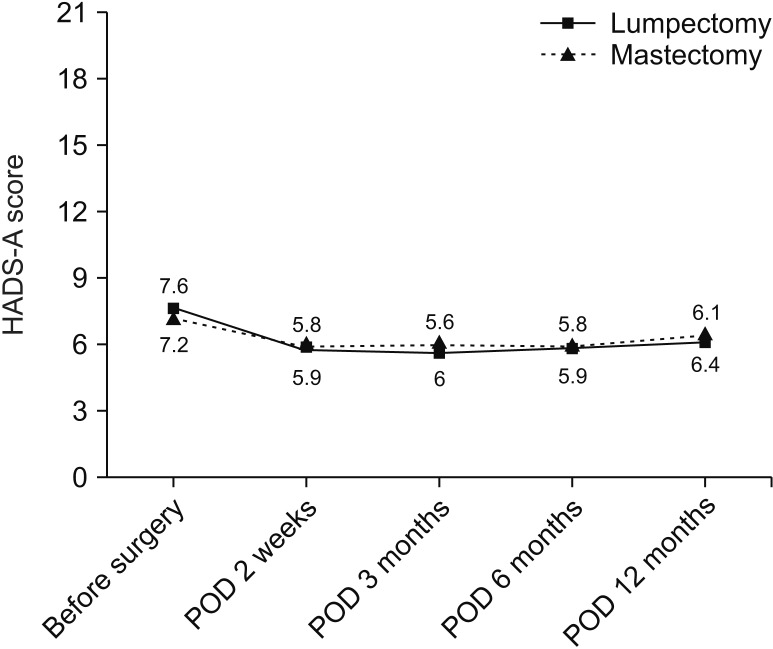
The anxiety level of the patients who underwent total mastectomy and partial mastectomy were not different (Fig. 5).
Previous studies have shown a diverse prevalence of anxiety, which depends on different criteria of the anxiety, diverse populations studied, differences in methodology used, and timing of measurement, ranging from about 20% to greater than 50% [1289101112]. In this study, we could see a high prevalence of anxiety after the diagnosis of breast cancer. The percentage of patients with anxiety in the normal range was just over half (55.5%). Pathologic or clinical levels of anxiety (scores more than 11) were shown in 21.6% patients.
In this analysis, we examined the temporal pattern of anxiety starting from cancer diagnosis. Many previous studies for anxiety in breast cancer patients were assessed crosssectionally during the courses of treatment with different cohorts [10]. Few researchers have reported anxiety level in relation to time progression; however, they did not show the baseline anxiety from diagnosis [21218]. Other previous studies demonstrated that level of anxiety was highest at diagnosis with improvement over time [1920], consistent with our data. However, these studies did not assess the anxiety level at immediate postoperative period, and could not show the effect of surgery itself on the anxiety.
Several studies showed distress trajectories including anxiety after cancer diagnosis [18]. Henselmans et al. [21] found that most breast cancer survivors within the first year postdiagnosis experienced no psychological distress (36.3%) or recovered to normal after completion of active treatment (33.3%), but small percentages of breast cancer survivors became distressed in the re-entry and survivorship phase (15.2%) or experienced persistent distress (15.2%) in the first year after breast cancer diagnosis. These are comparable with our study results in which 8.8% of patients became newly distressed in the re-entry periods (group 2) and 20.8% showed chronic anxiety (group 4) at month 12.
During the observation period of our cohort, there were 2 distinctive features. The first was rapid relief of anxiety after surgery. Patients were the most anxious before surgery, and this level of anxiety was sharply decreased after surgery. Understandably, this phenomenon was probably the fear of uncertainty about the cancer itself, treatment process, and outcomes. It could simply that surgery is a main relieving factor of anxiety; however, conversely, surgery itself is also a primary fear for patients after cancer diagnosis. Secondly, we could see the second rising of anxiety (re-entry) at 12 months. Most main adjuvant therapies were finished and the patients entered follow-up periods at this time. Several studies showed that anxiety generally decreased with time during the treatment period [8121822]. However, after completion of the treatment, the patients confront worries about leaving the health care system, uncertainty about the future, and the fear of recurrence [232425], and these could raise distress levels as in our results.
There have been many reports about the psychological distress associated factors in breast cancer [89101112222326]. Unlike these studies, in our study, socioeconomic factors, younger age, stage, social support, and mastectomy did not elevate anxiety. Adjuvant chemotherapy was preoperatively thought to be a risk factor of anxiety; however, after completion of chemotherapy, chemotherapy was not associated with elevated levels of anxiety. In our analysis, pain, and breast and arm symptoms were associated with persistent anxiety as in previous reports in which pain influences anxiety [81222].
There are some limitations to this study. This is a preliminary report with short-term follow-up data. Long-term follow-up results could provide the evidence and the reason for the reentry phenomenon. In addition to this, baseline distress, which was assessed preoperatively but not before diagnosis, did not reflect the real baseline (normal psychological) status of each person. If we have control cohorts (general population), the effect of breast cancer diagnosis on anxiety could be assessed more objectively. However, considering the result of previous research reporting that the prevalence of anxiety using HADS was 32.2% in generally healthy women [27], the breast cancer patients in our study seemed to suffer from anxiety more frequently after diagnosis. We needed also more information about previous mental health status because a past history of or pre-existing anxiety and/or depression before cancer diagnosis was known as an important predictor of anxiety [1928]. Regardless of these limitations, we have several important strengths. First is the design of this study. This is a prospective longitudinal study, and we could assess the psychological status from the preoperative period to 12 months. Also, our research was performed at clearly defined time points, including preoperative and immediate postoperative periods. Several prospective studies had much heterogeneity in assessment timing and did not assess the preoperative status.
In conclusion, anxiety disorder was common in patients who were diagnosed with breast cancer (44.5%). Surgery was a main relieving factor of anxiety and patients who finished their main adjuvant treatments experienced renewed anxiety. Surgeons should be the main detectors and care-givers for psychological distress in breast cancer patients during the time after diagnosis and completion of adjuvant therapy. To reduce persistent anxiety, taking care of physical symptoms is important.
Notes
Fund/Grant Support: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2017R1E1A1A0107764214).
References
1. Stark D, Kiely M, Smith A, Velikova G, House A, Selby P. Anxiety disorders in cancer patients: their nature, associations, and relation to quality of life. J Clin Oncol. 2002; 20:3137–3148. PMID: 12118028.

2. Taira N, Shimozuma K, Shiroiwa T, Ohsumi S, Kuroi K, Saji S, et al. Associations among baseline variables, treatment-related factors and health-related quality of life 2 years after breast cancer surgery. Breast Cancer Res Treat. 2011; 128:735–747. PMID: 21681445.

3. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000; 160:2101–2107. PMID: 10904452.
4. Greer JA, Pirl WF, Park ER, Lynch TJ, Temel JS. Behavioral and psychological predictors of chemotherapy adherence in patients with advanced non-small cell lung cancer. J Psychosom Res. 2008; 65:549–552. PMID: 19027443.

5. Groenvold M, Petersen MA, Idler E, Bjorner JB, Fayers PM, Mouridsen HT. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat. 2007; 105:209–219. PMID: 17203386.

6. Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009; 7:102. PMID: 20030832.

7. Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer pat ients: a meta-analysis. Cancer. 2009; 115:5349–5361. PMID: 19753617.
8. Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005; 330:702. PMID: 15695497.

9. So WK, Marsh G, Ling WM, Leung FY, Lo JC, Yeung M, et al. Anxiety, depression and quality of life among Chinese breast cancer patients during adjuvant therapy. Eur J Oncol Nurs. 2010; 14:17–22. PMID: 19734087.

10. Ho SS, So WK, Leung DY, Lai ET, Chan CW. Anxiety, depression and quality of life in Chinese women with breast cancer during and after treatment: a comparative evaluation. Eur J Oncol Nurs. 2013; 17:877–882. PMID: 23727448.

11. Schubart JR, Emerich M, Farnan M, Stanley Smith J, Kauffman GL, Kass RB. Screening for psychological distress in surgical breast cancer patients. Ann Surg Oncol. 2014; 21:3348–3353. PMID: 25034820.

12. Saboonchi F, Petersson LM, Wennman-Larsen A, Alexanderson K, Brannstrom R, Vaez M. Changes in caseness of anxiety and depression in breast cancer patients during the first year following surgery: patterns of transiency and severity of the distress response. Eur J Oncol Nurs. 2014; 18:598–604. PMID: 24997517.

13. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983; 67:361–370. PMID: 6880820.

14. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. 2002; 52:69–77. PMID: 11832252.
15. Oh SM, Min KJ, Park DB. A study on the standardization of the hospital anxiety and depression scale for koreans: a comparison of normal, depressed and anxious groups. J Korean Neuropsychiatr Assoc. 1999; 38:289–296.
16. Sprangers MA, Groenvold M, Arraras JI, Franklin J, te Velde A, Muller M, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996; 14:2756–2768. PMID: 8874337.

17. Yun YH, Bae SH, Kang IO, Shin KH, Lee R, Kwon SI, et al. Cross-cultural application of the Korean version of the European Organization for Research and Treatment of Cancer (EORTC) Breast-Cancer-Specific Quality of Life Questionnaire (EORTC QLQ-BR23). Support Care Cancer. 2004; 12:441–445. PMID: 15088137.
18. Boyes AW, Girgis A, D'Este CA, Zucca AC, Lecathelinais C, Carey ML. Prevalence and predictors of the short-term trajectory of anxiety and depression in the first year after a cancer diagnosis: a populationbased longitudinal study. J Clin Oncol. 2013; 31:2724–2729. PMID: 23775970.

19. Stafford L, Judd F, Gibson P, Komiti A, Mann GB, Quinn M. Anxiety and depression symptoms in the 2 years fol lowing diagnosis of breast or gynaecologic cancer: prevalence, course and determinants of outcome. Support Care Cancer. 2015; 23:2215–2224. PMID: 25559036.
20. Kyranou M, Puntillo K, Dunn LB, Aouizerat BE, Paul SM, Cooper BA, et al. Predictors of initial levels and trajectories of anxiety in women before and for 6 months after breast cancer surgery. Cancer Nurs. 2014; 37:406–417. PMID: 24633334.

21. Henselmans I, Helgeson VS, Seltman H, de Vries J, Sanderman R, Ranchor AV. Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis. Health Psychol. 2010; 29:160–168. PMID: 20230089.

22. Schwarz R, Krauss O, Hockel M, Meyer A, Zenger M, Hinz A. The course of anxiety and depression in patients with breast cancer and gynaecological cancer. Breast Care (Basel). 2008; 3:417–422. PMID: 21048913.

23. Karakoyun-Celik O, Gorken I, Sahin S, Orcin E, Alanyali H, Kinay M. Depression and anxiety levels in woman under follow-up for breast cancer: relationship to coping with cancer and quality of life. Med Oncol. 2010; 27:108–113. PMID: 19225913.

24. Koch L, Jansen L, Brenner H, Arndt V. Fear of recurrence and disease progression in long-term (≥ 5 years) cancer survivors--a systematic review of quantitative studies. Psychooncology. 2013; 22:1–11.
25. Jefford M, Karahalios E, Pollard A, Baravelli C, Carey M, Franklin J, et al. Survivorship issues following treatment completion--results from focus groups with Australian cancer survivors and health professionals. J Cancer Surviv. 2008; 2:20–32. PMID: 18648984.

26. Yang H, Brand JS, Fang F, Chiesa F, Johansson AL, Hall P, et al. Time-dependent risk of depression, anxiety, and stress-related disorders in patients with invasive and in situ breast cancer. Int J Cancer. 2017; 140:841–852. PMID: 27859142.
27. Kim SH, Kang S, Kim YM, Kim BG, Seong SJ, Cha SD, et al. Prevalence and predictors of anxiety and depression among cervical cancer survivors in Korea. Int J Gynecol Cancer. 2010; 20:1017–1024. PMID: 20683411.

28. Stafford L, Komiti A, Bousman C, Judd F, Gibson P, Mann GB, et al. Predictors of depression and anxiety symptom trajectories in the 24 months following diagnosis of breast or gynaecologic cancer. Breast. 2016; 26:100–105. PMID: 27017248.

SUPPLEMENTARY MATERIALS
Supplementary questionnaires can be found via https://doi.org/10.4174/astr.2020.98.5.215
Supplementary Table 3
The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire - the breast cancer specific module (EORTC BR-23) English version
Supplementary Table 4
The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire - the breast cancer specific module (EORTC BR-23) Korean version
Fig. 2
Temporal patterns of anxiety symptom severity by mean anxiety dimension in Hospital Anxiety Depression Scale (HADS-A) score from diagnosis to 12 months. POD, postoperative day. *P < 0.001.
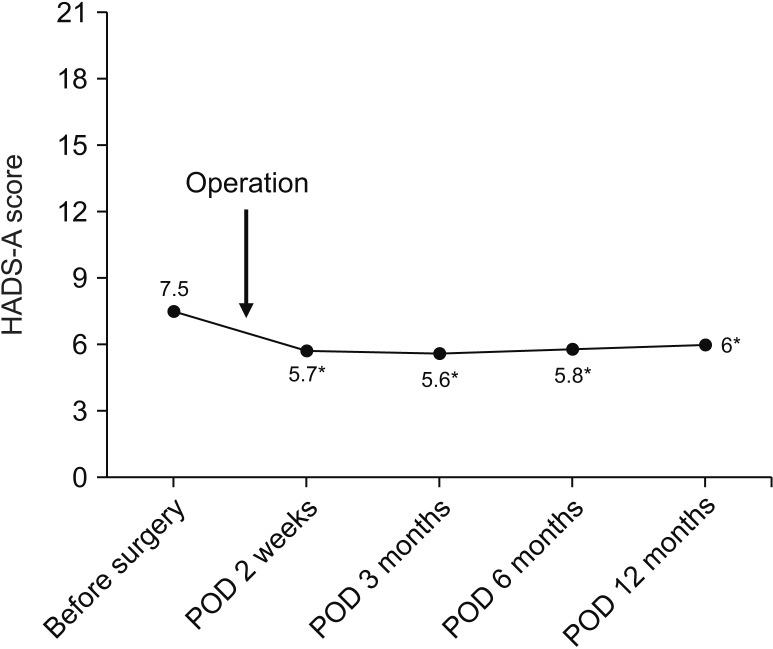
Fig. 3
Prevalence of anxiety at postoperative day 12 months according to the baseline anxiety (n = 250).
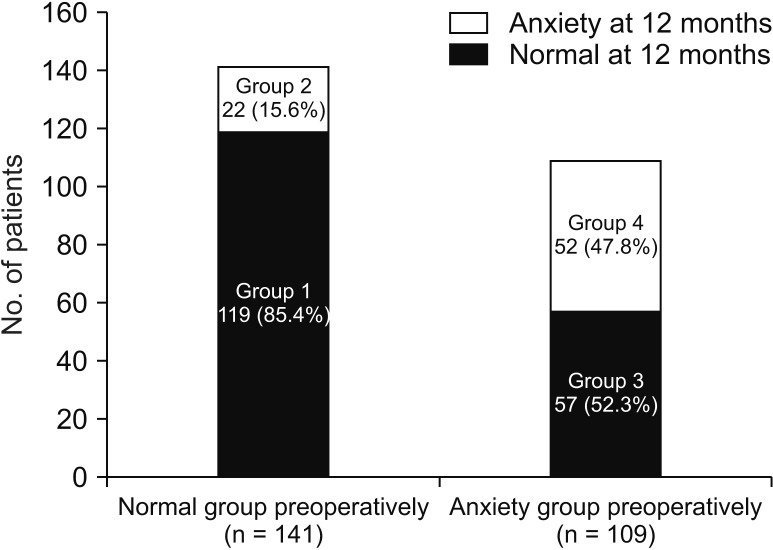
Fig. 4
Temporal pattern of anxiety symptom severity by mean anxiety dimension in Hospital Anxiety Depression Scale (HADS-A) score of each group over time (group 1, normal; group 2, newly developed anxiety; group 3, resolved anxiety; and group 4, persistent anxiety). POD, postoperative day.
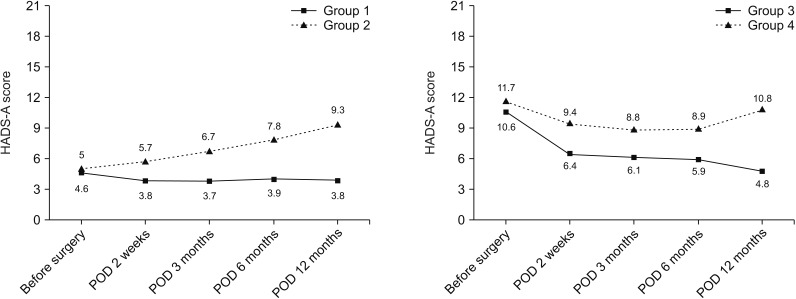
Fig. 5
Comparison of anxiety level with regard to follow-up duration by breast cancer operation type. HADS-A, anxiety dimension in Hospital Anxiety Depression Scale; POD, postoperative day.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download