Abstract
Purpose
Under the South Korea's unique health insurance structure, any new surgical technology must be evaluated first by the government in order to consider whether that particular technology can be applied to patients for further clinical trials as categorized as ‘New Health Technology,’ then potentially covered by the insurance sometime later. The aim of this meta-analysis was to assess the safety and efficacy of transanal total mesorectal excision (TaTME) for rectal cancer, activated by the National Evidence-based Healthcare Collaborating Agency (NECA) TaTME committee.
Methods
We systematically searched Ovid-MEDLINE, Ovid-Embase, Cochrane, and Korean databases (from their inception until August 31, 2019) for studies published that compare TaTME with laparoscopic total mesorectal excision (LaTME). End-points included perioperative and pathological outcomes.
Results
Sixteen cohort studies (7 for case-matched studies) were identified, comprising 1,923 patients (938 TaTMEs and 985 LaTMEs). Regarding perioperative outcomes, the conversion rate was significantly lower in TaTME (risk ratio, 0.19; 95% confidence interval, 0.11–0.34; P < 0.001); whereas other perioperative outcomes were similar to LaTME. There were no statistically significant differences in pathological results between the 2 procedures.
Conclusion
Our meta-analysis showed comparable results in preoperative and pathologic outcomes between TaTME and LaTME, and indicated the benefit of TaTME with low conversion. Extensive evaluations of well-designed, multicenter randomized controlled trials are required to come to unequivocal conclusions, but the results showed that TaTME is a potentially beneficial technique in some specific cases. This meta-analysis suggests that TaTME can be performed for rectal cancer patients as a ‘New Health Technology’ endorsed by NECA in South Korea.
Colorectal cancer is the third most common diagnosis and the third-leading cause of death worldwide [1]. Total mesorectal excision (TME) was first described by Heald and Ryall in 1982 [2], and it has become the standard treatment for rectal cancer by effectively reducing recurrence, improving quality of life, and prolonging survival [3]. Laparoscopic surgery has replaced open surgery, allowing favorable short-term outcomes, particularly for pain reduction, reduced blood loss, and rapid recovery [4]. Laparoscopic surgery has been widely accepted and used for minimally invasive surgical procedures; however, conversions from laparoscopic TME (LaTME) to open procedure have been common in patients with rectal cancer [5]. The main reasons for the unsatisfactory conversion rate were the patient characteristics of male sex (because of the narrow pelvis) and the high body mass index (BMI) [67]. Additionally, high incidence of positive resection margin and poor quality of TME specimen, which are factors known as poor oncologic outcomes in deep narrow pelvis, exist [8].
To address the aforementioned limitations, transanal TME (TaTME) was first described by Sylla et al. [9]. In several studies, this “bottom-up” approach for mid-low rectal cancer has been proposed as a safe and effective technique for patients by demonstrating the benefits of more precise excision under appropriate visualization and potentially improving specimen quality and resection margins [101112]. However, it still remains controversial because its oncological feasibility and safety have not yet been validated by large randomized controlled trials (RCTs). Recent studies reported that the TaTME approach showed unfavorable local recurrence rates and a higher risk of anastomotic leak than a nontransanal approach [1314]. TaTME also seems to be associated with substantial morbidities such as urethral and other urologic injuries [15]. It is suggested that these complications are related to the surgeon's lack of surgical training or learning curves [1617].
The national health insurance system of South Korea is unique public health insurance where all citizens are forced to sign in, and all insurance policyholders are burdened with the duty to pay for the insurance. By the unique structure of South Korea's health insurance system, if a new medical procedure doesn't pass the New Health Technology Assessment (nHTA), it cannot be covered by the health insurance, thus not being available to policyholders even as an uncovered procedure. Therefore, nHTA is an essential step undertaken to evaluate whether a procedure's clinical safety/efficacy is appropriate to be used on insurance policyholders through a systematic review.
TaTME is a new surgical technique in which the technical method of conventional LaTME has been changed and is subject to nHTA. Thus, a systematic review and meta-analysis of the current data from the latest and most convincing studies was conducted, comparing the safety and efficacy between TaTME and LaTME for nHTA by the National Evidence-based Healthcare Collaborating Agency (NECA) committee.
This study was conducted in accordance with the PRISMA (preferred reporting items for systematic reviews and metaanalyses) guidelines [18]. The protocol was registered in PROSPERO under the number CRD42021230076. Comprehensive searches were performed through the databases of MEDLINE, Embase, Cochrane Library, and Korean databases (KoreaMed, KMbase, KISTI, KISS, and RISS) from their inception until August 31, 2019. The MEDLINE and Embase databases were searched using the following terms with Boolean operators: (rectal neoplasms OR rectal tumor OR rectal cancer OR colorectal neoplasms OR colorectal tumor OR colorectal cancer) AND (transanal total mesorectal excision OR TaTME OR Ta-TME OR transanal minimally invasive surgery OR TAMIS OR transanal endoscopic surgery OR transanal rectal resection). The Cochrane database was searched using the following keyword: transanal total mesorectal excision. The Korean database was searched using the following terms with Boolean operators: neoplasms AND transanal total mesorectal excision.
In accordance with PICOS (patient, intervention, comparator, outcome, and study design) criteria, studies were included if they met the following inclusion criteria: (1) patients diagnosed with rectal cancer; (2) patients underwent either TaTME or LaTME; (3) at least 1 of the following perioperative outcomes or pathological data were available; and (4) studies were cohort studies. The exclusion criteria were as follows: (1) reviews, letters, editorials, commentaries, conference abstract, and clinical reports; (2) studies with sample size below 20 in each group; (3) languages other than English; (4) inappropriate data; (5) duplicate patient series; (6) inadequate technique for intervention or comparator; and (7) nonhuman researches.
The following data were extracted by the 2 reviewers (SHK and YIJ). (1) Demographic data: study design, sex, BMI, age, American Society of Anesthesiologists physical status classification, tumor location, and neoadjuvant treatment; (2) Perioperative outcomes: operation time, intraoperative blood loss, conversion rate, hospital stay, readmission, reoperation, diverting ileostomy, major complication (Clavien-Dindo classification III–V), anastomotic leakage, intestinal obstruction, ureter or urethral injury, urinary retention, urinary tract infection, and mortality; (3) Pathological outcomes: circumferential resection margin (CRM) and distal resection margin (DRM) involvement, length of CRM and DRM, incompleteness of mesorectum, and harvested lymph nodes. The methodological quality and risk of bias of the included studies were assessed using the Newcastle-Ottawa quality assessment scale [19]. Any disagreements were settled by consensus-based discussion between the 2 reviewers (SHK and YIJ).
Inappropriate techniques described in the exclusion criteria are defined as all procedures except interventional (TaTME) or comparative procedures (LaTME). The location of the tumor is categorized as low (0–5 cm from the anal verge), middle (5.1–10 cm from the anal verge), and high (10.1–15 cm from the anal verge). Conversion in LaTME was defined when the procedure was completed with open surgery. Conversion in TaTME was defined as a case in which the procedure was completed by open surgery, or the TME was performed by the transanal approach but the conversion occurred at the transabdominal phase. Mesorectal resection quality was scored using 3 grades—complete, nearly complete, or incomplete, as defined by Quirke et al. [20].
Meta-analyses were performed with Review Manager 5.3 software (Cochrane Collaboration, Oxford, UK). Dichotomous data were pooled as risk ratios (RRs) with 95% confidence intervals (CIs) using the Mantel-Haenszel method, which can avoid biased estimates by incorporating evidence from single zero studies without requiring the standard continuity correction [21]. Mean differences (MDs) and 95% CI were pooled for continuous variables using the inverse variance method. If the median and range were reported instead of the mean and standard deviation, these values were estimated using the method devised by Hozo et al. [22]. The Q test and I2 statistic were used to evaluate heterogeneity among studies. The Cochrane Handbook for Systematic Reviews of Interventions provides a rule of thumb for interpreting the I2 statistic; that is I2 ≤ 40% may indicate unimportant heterogeneity, 30% ≤ I2 ≤ 60% may represent moderate heterogeneity, 50% ≤ I2 ≤ 90% may represent substantial heterogeneity, and 70% ≤ I2 ≤ 100% implies that heterogeneity may be considerable. A Cochrane Q statistical P < 0.100 and/or I2 > 50% was taken to indicate significant heterogeneity, in which case a random-effects model was applied [23]. Otherwise, a fixed-effects model was employed. Funnel plots were used in meta-analysis to visually detect the presence of publication bias.
As a result of the literature search, 1,940 studies were identified. Among the search results obtained through the online databases in the first screening, 534 duplicate articles were removed. After that, through the review of the abstracts, 857 articles that did not fit the language and article type were excluded. The remaining articles were fully checked to ensure they met the inclusion criteria, and 533 additional articles were excluded. Finally, 16 cohort studies met all the inclusion criteria and none of the exclusion criteria (Fig. 1) [24252627282930313233343536373839]. Seven of the cohort studies were case-matched studies comparing TaTME with LaTME for rectal cancer [25282930313638]. The patient demographics, clinical characteristics, and quality assessment scores are shown in Table 1 and Supplementary Table 1.
A total of 12 studies [242526272830313234353638] reported operation time in 946 patients, and we found no statistically significant difference between TaTME and LaTME groups (MD, 7.52; 95% CI, −10.03–25.07; P = 0.400) (Fig. 2A). Perdawood et al. [35] and Fernández-Hevia et al. [24] reported a 2-team approach as standard; Alamili et al. [32], Chen et al. [34], Roodbeen et al. [36], Sparreboom et al. [38], Chang and Kiu [28], and Mege et al. [30] used a 1-team approach. Persiani et al. [31], Lelong et al. [37], Chen et al. [25], and Rasulov et al. [26] used both a 1- and 2-team approach. There was a notable heterogeneity between included studies (I2 = 88%, P < 0.001).
Intraoperative blood loss repor ted in 6 studies [252628323435] that investigated 462 patients (Fig. 2B). There was no statistically significant difference between groups (MD, −8.37; 95% CI, −27.82–11.08; P = 0.410), and moderate heterogeneity was existed (I2 = 48%, P = 0.090).
Data on conversion rate were extracted from 13 studies [24252627282930313234353638] assessing 1,738 patients (Fig. 2C). Upon analysis, the TaTME group displayed significant lower conversion rate compared to the LaTME group (RR, 0.19; 95% CI, 0.11–0.34; P < 0.001). There was no heterogeneity between included studies (I2 = 0%, P = 0.460).
Twelve studies [242526272829303132343638] assessed hospital stay in 1,680 patients (Fig. 3A). Pooled analysis indicated that the result was not statistically significant between the 2 groups (MD, −0.51; 95% CI, −1.43–0.41; P = 0.280). Substantial heterogeneity existed in hospital stay (I2 = 88%, P < 0.001).
A total of 7 studies [24252729323638] reported data on readmission in 1,326 patients (Fig. 3B). There was no statistically significant difference between the 2 groups (RR, 0.90; 95% CI, 0.70–1.15; P = 0.400), and moderate heterogeneity existed (I2 = 49%, P = 0.070).
Five studies [2425273238] that evaluated 452 patients were pooled for analysis of reoperation (Fig. 3C). The difference was not statistically significant between the 2 groups (RR, 0.86; 95% CI, 0.45–1.61; P = 0.630). There was no heterogeneity between included studies (I2 = 0%, P = 0.470).
Analysis of the major complication (Clavien-Dindo classification III–V) was performed based on 9 studies [242627303136373839] evaluating 629 patients (Fig. 4A). There was no statistically detected difference between the groups (RR, 0.75; 95% CI, 0.50–1.13; P = 0.170). Heterogeneity in this regard was not significant (I2 = 0%, P = 0.640).
Data on anastomotic leakage were available in 9 studies [242829303132343638], which assessed 1,196 patients (Fig. 4B). We found that there was no statistically significant difference between the 2 groups (RR, 1.32; 95% CI, 0.96–1.82; P = 0.090), with no heterogeneity (I2 = 26%, P = 0.210).
Intestinal obstruction was investigated in 8 studies [2425283031323438], and remained unchanged between the 2 groups (RR, 1.01; 95% CI, 0.59–1.72; P = 0.970) (Fig. 4C). There was no heterogeneity between included studies (I2 = 0%, P = 0.940).
Moreover, the occurrence of other complications such as diverting ileostomy (RR, 1.20; 95% CI, 0.87–1.65; P = 0.270; I2 = 85%), mortality within 30 days (RR, 0.40; 95% CI, 0.06–2.73; P = 0.350; I2 = 0%), ureter or urethral injury (RR, 0.83; 95% CI, 0.10–6.63; P = 0.860; I2 = 0%), urinary retention (RR, 1.23; 95% CI, 0.04–38.46; P = 0.910; I2 = 74%), and urinary tract infection (RR, 1.08; 95% CI, 0.29–4.11; P = 0.910; I2 = 0%) were similar between the 2 groups (Supplementary Fig. 1, 2).
Fifteen studies [242526272829303133343536373839] that assessed 1,863 patients reported on CRM involvement (Fig. 5A). The results showed that CRM involvement was similar between the 2 groups (RR, 0.73; 95% CI, 0.48–1.10; P = 0.130), and no heterogeneity was detected between included studies (I2 = 0%; P = 0.750).
The length of CRM was reported in 4 studies [24323536] that investigated 274 patients (Fig. 5B). The comparison between the 2 groups resulted in a difference that was not statistically significant (MD, 1.64; 95% CI, −1.32–4.60; P = 0.280), but there was a notable heterogeneity (I2 = 97%; P < 0.001).
Seven studies [26273035363738] assessed 467 patients and reported on DRM involvement (Fig. 5C). DRM involvement is not significantly different between the 2 groups (RR, 0.60; 95% CI, 0.32–1.15; P = 0.120), with no significant between-study heterogeneity (I2 = 0%, P = 0.880).
A total of 9 studies [242528303132343536] reported length of DRM in 733 patients (Fig. 5D), which was not significantly different between the 2 groups (MD, 1.88; 95% CI, −2.96–6.73; P = 0.450). Statistical heterogeneity among the studies was high (I2 = 87%, P < 0.001).
Eleven studies [2426273031323335363739] assessed 736 patients and provided data on incompleteness of mesorectum (Fig. 5E). Pooled analysis showed that the incompleteness of mesorectum was equivalent between the 2 groups (RR, 1.00; 95% CI, 0.70–1.45; P = 0.980). There was no heterogeneity between included studies (I2 = 10%, P = 0.350).
Symmetrical funnel plots of the conversion rate, major complication (Clavien-Dindo classification III–V), CRM involvement, and incompleteness of mesorectum suggested no publication bias exists in the meta-analysis (Supplementary Fig. 3), and all of the studies were within the 95% CIs.
In the present systematic review and meta-analysis, we evaluated the bottom-up approach of TaTME compared to conventional LaTME with respect to perioperative and pathological outcomes. Our meta-analysis shows that TaTME has a significantly lower conversion rate compared to LaTME. Open surgical conversion is one of the major problems in laparoscopic surgery for rectal cancer. Conversion rates in laparoscopic surgery depend on several factors such as patient-related factors, surgeon-related factors, and procedural factors. Unlike other factors, patient-related factors are beyond the control of the surgeon. Therefore, to determine the success of the laparoscopic approach, it is important to consider patient-related factors such as sex, obesity, tumor stage, and previous abdominal operation [404142]. Furthermore, patient selection based on accurate tumor characteristics can reduce conversion rates [43]. TaTME has emerged as a feasible alternative surgical option for conventional laparoscopic surgery, particularly in patients with obesity, narrow pelvis, or deep anterior rectal tumors [44]. Notably, our pooled analysis showed significantly lower conversion rates in patients undergoing TaTME (Fig. 2C), suggesting that the transanal approach can overcome limitations on patient-related factors.
While several studies have reported that the 2-team approach of the TaTME procedure has shorter operation times, but this issue is one of the major controversies currently being raised. In the studies included in our meta-analysis, a 2-team approach [2435], a 1-team approach [283032343638], and both approaches [252731] were performed. The 2-team approach tended to have shorter operation times compared to the 1-team approach, but there were not enough literatures available for subgroup analysis. TaTME's approach may be carried out differently depending on the circumstances, such as the national healthcare system and health insurance policies or the status of medical personnel in hospitals. In this regard, a recent study reported cost analysis, including surgical supplies along with surgical outcomes [45]. When discussing TaTME's approach in the future, surgical outcomes such as operation time and cost analysis also need to be considered.
Anastomotic leakage is a major complication of colorectal surgery causing increased mortality, the incidence of which has persisted over the last years [4647]. Recently, an international multicenter cohort study reported that TaTME had a higher risk of anastomotic leakage than LaTME [13]. On the other hand, a recent cohort study of the incidence of anastomosis and learning curves after TaTME reported that surgeon proficiency can have a positive effect on the occurrence of anastomotic leakage. Our findings on the perioperative outcomes showed that LaTME tended to have a higher rate of anastomotic leakage than TaTME, but there was no statistically significant difference (Fig. 4B). Since a wide range of anastomotic leak rates depends on the definition, clinical setting, and follow-up period [29], subgroup analysis of the related factors needs to be conducted through large-scale study to draw clear conclusions.
A Norwegian research group noted that TaTME could increase the risk of local recurrence [48]. The results showed local recurrence of a new pattern characterized by rapid, multifocal growth in the pelvic cavity and sidewalls early after TaTME, which led to a nationwide discontinuance of TaTME [4849]. It should be noted that although there are concerns about the risk of local recurrence, conclusions cannot be drawn due to insufficient numbers and lack of long-term oncological follow-up from the national multicenter study. These results further suggest that structured training with proctorship experienced proponents is essential.
One of the potential benefits of TaTME is improved specimen quality, defined as CRM involvement, DRM distance, and mesorectal completeness. These pathological outcomes are the most potent prognostic factors predicting local recurrence [5051]. Fifteen studies involving 1,863 patients showed a tendency to a reduced CRM positive status of TaTME in comparison with LaTME (Fig. 5A). Regarding the DRM distance, 4 of 9 studies reported that TaTME showed a longer DRM compared to LaTME [24253135] (Fig. 5D). Furthermore, incompleteness of mesorectum in TaTME was similar to the results from LaTME (Fig. 5E). However, contrary to the results reported in the individual studies, meta-analysis showed TaTME did not lead to significant improvement in pathological outcomes when compared to LaTME.
Among the surgical outcomes of TaTME and LaTME, studies evaluating the length of CRM and DRM showed considerable heterogeneity, and there is one possible explanation for the significant heterogeneity. We used the method of estimating the median value as the mean value for meta-analysis, and it seems that heterogeneity occurred in this process. In the study of Alamili et al. [32], the median was similar between the 2 groups, but the difference in sample size resulted in a large difference in the estimate of the mean value. The main limitation of this method is that the outcome distribution is assumed to be normal, and new methods have been developed to overcome this limitation [52]. However, since any method may make an unsuitable assumption, results should be interpreted with additional explanations.
In conclusion, this updated systematic review and meta-analysis showed that TaTME has potential advantages in surgical outcomes, suggesting that TaTME can be performed as a New Health Technology for rectal cancer patients in South Korea. However, since this study was conducted based on limited evidence, the results of a well-designed multicenter RCTs need to be further evaluated to apply our conclusions extensively; and expertise and training to safely perform TaTME should also be considered.
Notes
Fund/Grant Support: This study is supported by the National Evidence-based Healthcare Collaborating Agency in South Korea (the Health Technology Assessment Report #HTA-2020-10).
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. PMID: 30207593.

2. Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery-the clue to pelvic recurrence? Br J Surg. 1982; 69:613–616. PMID: 6751457.

3. Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998; 133:894–899. PMID: 9711965.
4. Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005; 6:477–484. PMID: 15992696.
5. Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005; 365:1718–1726. PMID: 15894098.

6. Penninckx F, Kartheuser A, Van de Stadt J, Pattyn P, Mansvelt B, Bertrand C, et al. Outcome following laparoscopic and open total mesorectal excision for rectal cancer. Br J Surg. 2013; 100:1368–1375. PMID: 23939849.

7. Lujan J, Valero G, Hernandez Q, Sanchez A, Frutos MD, Parrilla P. Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg. 2009; 96:982–989. PMID: 19644973.

8. Mizrahi I, Sands DR. Transanal total mesorectal excision for rectal cancer: a review. Ann Laparosc Endosc Surg. 2017; 2:144.

9. Sylla P, Rattner DW, Delgado S, Lacy AM. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010; 24:1205–1210. PMID: 20186432.

10. Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, et al. Transanal total mesorectal excision: international registry results of the first 720 cases. Ann Surg. 2017; 266:111–117. PMID: 27735827.
11. de Lacy FB, van Laarhoven JJ, Pena R, Arroyave MC, Bravo R, Cuatrecasas M, et al. Transanal total mesorectal excision: pathological results of 186 patients with mid and low rectal cancer. Surg Endosc. 2018; 32:2442–2447. PMID: 29101570.

12. Abbott SC, Stevenson AR, Bell SW, Clark D, Merrie A, Hayes J, et al. An assessment of an Australasian pathway for the introduction of transanal total mesorectal excision (taTME). Colorectal Dis. 2018; 20:O1–O6. PMID: 29165862.

13. 2017 European Society of Coloproctology (ESCP) collaborating group. An international multicentre prospective audit of elective rectal cancer surgery; operative approach versus outcome, including transanal total mesorectal excision (TaTME). Colorectal Dis. 2018; 20 Suppl 6:33–46. PMID: 30255642.
14. Wasmuth HH, Faerden AE, Myklebust TÅ, Pfeffer F, Norderval S, Riis R, et al. Transanal total mesorectal excision for rectal cancer has been suspended in Norway. Br J Surg. 2020; 107:121–130. PMID: 31802481.

15. Sylla P, Knol JJ, D'Andrea AP, Perez RO, Atallah SB, Penna M, et al. Urethral injury and other urologic injuries during transanal total mesorectal excision: an international collaborative study. Ann Surg. 2021; 274:e115–e125. PMID: 31567502.
16. Caycedo-Marulanda A, Verschoor CP. Experience beyond the learning curve of transanal total mesorectal excision (taTME) and its effect on the incidence of anastomotic leak. Tech Coloproctol. 2020; 24:309–316. PMID: 32112245.

17. Persiani R, Agnes A, Belia F, D'Ugo D, Biondi A. The learning curve of TaTME for mid-low rectal cancer: a comprehensive analysis from a five-year institutional experience. Surg Endosc. 2020; 10. 26. [Epub]. DOI: 10.1007/s00464-020-08115-0.

18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare inter vent ions: expl anat ion and elaboration. BMJ. 2009; 339:b2700. PMID: 19622552.
19. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010; 25:603–605. PMID: 20652370.

20. Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009; 373:821–828. PMID: 19269520.

21. Efthimiou O. Practical guide to the meta-analysis of rare events. Evid Based Ment Health. 2018; 21:72–76. PMID: 29650528.

22. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005; 5:13. PMID: 15840177.

23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560. PMID: 12958120.

24. Fernández-Hevia M, Delgado S, Castells A, Tasende M, Momblan D, Díaz del Gobbo G, et al. Transanal total mesorectal excision in rectal cancer: short-term outcomes in comparison with laparoscopic surgery. Ann Surg. 2015; 261:221–227. PMID: 25185463.
25. Chen CC, Lai YL, Jiang JK, Chu CH, Huang IP, Chen WS, et al. Transanal total mesorectal excision versus laparoscopic surgery for rectal cancer receiving neoadjuvant chemoradiation: a matched case-control study. Ann Surg Oncol. 2016; 23:1169–1176. PMID: 26597369.

26. Rasulov AO, Mamedli ZZ, Gordeyev SS, Kozlov NA, Dzhumabaev HE. Short-term outcomes after transanal and laparoscopic total mesorectal excision for rectal cancer. Tech Coloproctol. 2016; 20:227–234. PMID: 26794213.

27. Lelong B, Meillat H, Zemmour C, Poizat F, Ewald J, Mege D, et al. Short- and mid-term outcomes after endoscopic transanal or laparoscopic transabdominal total mesorectal excision for low rectal cancer: a single institutional case-control study. J Am Coll Surg. 2017; 224:917–925. PMID: 28024946.

28. Chang TC, Kiu KT. Transanal total mesorectal excision in lower rectal cancer: comparison of short-term outcomes with conventional laparoscopic total mesorectal excision. J Laparoendosc Adv Surg Tech A. 2018; 28:365–369. PMID: 29190178.

29. Detering R, Roodbeen SX, van Oostendorp SE, Dekker JT, Sietses C, Bemelman WA, et al. Three-year nationwide experience with transanal total mesorectal excision for rectal cancer in the Netherlands: a propensity score-matched comparison with conventional laparoscopic total mesorectal excision. J Am Coll Surg. 2019; 228:235–244. PMID: 30639298.

30. Mege D, Hain E, Lakkis Z, Maggiori L, Prost À, Panis Y. Is trans-anal total mesorectal excision really safe and better than laparoscopic total mesorectal excision with a perineal approach first in patients with low rectal cancer?: a learning curve with case-matched study in 68 patients. Colorectal Dis. 2018; 20:O143–O151. PMID: 29693307.

31. Persiani R, Biondi A, Pennestrì F, Fico V, De Simone V, Tirelli F, et al. Transanal total mesorectal excision vs laparoscopic total mesorectal excision in the treatment of low and middle rectal cancer: a propensity score matching analysis. Dis Colon Rectum. 2018; 61:809–816. PMID: 29771810.

32. Alamili M, Levic K, Kanstrup K, Bisgaard T, Bulut O. Inflammatory response after transanal total mesorectal excision. Dan Med J. 2019; 66:A5555. PMID: 31256779.
33. Bjoern MX, Nielsen S, Perdawood SK. Quality of life after surgery for rectal cancer: a comparison of functional outcomes after transanal and laparoscopic approaches. J Gastrointest Surg. 2019; 23:1623–1630. PMID: 30603861.

34. Chen YT, Kiu KT, Yen MH, Chang TC. Comparison of the short-term outcomes in lower rectal cancer using three different surgical techniques: transanal total mesorectal excision (TME), laparoscopic TME, and open TME. Asian J Surg. 2019; 42:674–680. PMID: 30318319.

35. Perdawood SK, Warnecke M, Bjoern MX, Eiholm S. The pattern of defects in mesorectal specimens: is there a difference between transanal and laparoscopic approaches? Scand J Surg. 2019; 108:49–54. PMID: 29966503.

36. Roodbeen SX, Penna M, Mackenzie H, Kusters M, Slater A, Jones OM, et al. Transanal total mesorectal excision (TaTME) versus laparoscopic TME for MRI-defined low rectal cancer: a propensity score-matched analysis of oncological outcomes. Surg Endosc. 2019; 33:2459–2467. PMID: 30350103.

37. Rubinkiewicz M, Zarzycki P, Witowski J, Pisarska M, Gajewska N, Torbicz G, et al. Functional outcomes after resections for low rectal tumors: comparison of transanal with laparoscopic total mesorectal excision. BMC Surg. 2019; 19:79. PMID: 31277628.

38. Sparreboom CL, Komen N, Rizopoulos D, van Westreenen HL, Doornebosch PG, Dekker JW, et al. Transanal total mesorectal excision: how are we doing so far? Colorectal Dis. 2019; 21:767–774. PMID: 30844130.

39. Veltcamp Helbach M, Koedam TW, Knol JJ, Velthuis S, Bonjer HJ, Tuynman JB, et al. Quality of life after rectal cancer surgery: differences between laparoscopic and transanal total mesorectal excision. Surg Endosc. 2019; 33:79–87. PMID: 29967994.

40. Thorpe H, Jayne DG, Guillou PJ, Quirke P, Copeland J, Brown JM, et al. Patient factors influencing conversion from laparoscopical ly assisted to open surgery for colorectal cancer. Br J Surg. 2008; 95:199–205. PMID: 17696215.
41. Pandya S, Murray JJ, Coller JA, Rusin LC. Laparoscopic colectomy: indications for conversion to laparotomy. Arch Surg. 1999; 134:471–475. PMID: 10323418.
42. Pikarsky AJ, Saida Y, Yamaguchi T, Martinez S, Chen W, Weiss EG, et al. Is obesity a high-risk factor for laparoscopic colorectal surgery? Surg Endosc. 2002; 16:855–858. PMID: 11997837.

43. Veldkamp R, Gholghesaei M, Bonjer HJ, Meijer DW, Buunen M, Jeekel J, et al. Laparoscopic resection of colon cancer: consensus of the European Association of Endoscopic Surgery (EAES). Surg Endosc. 2004; 18:1163–1185. PMID: 15457376.

44. Rouanet P, Mourregot A, Azar CC, Carrere S, Gutowski M, Quenet F, et al. Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum. 2013; 56:408–415. PMID: 23478607.
45. Di Candido F, Carvello M, Keller DS, Vanni E, Maroli A, Montroni I, et al. A comparative cost analysis of transanal and laparoscopic total mesorectal excision for rectal cancer. Updates Surg. 2021; 73:85–91. PMID: 32929690.

46. Krarup PM, Jorgensen LN, Andreasen AH, Harling H. Danish Colorectal Cancer Group. A nationwide study on anastomotic leakage after colonic cancer surgery. Colorectal Dis. 2012; 14:e661–e667. PMID: 22564292.

47. Paun BC, Cassie S, MacLean AR, Dixon E, Buie WD. Postoperative complications following surgery for rectal cancer. Ann Surg. 2010; 251:807–818. PMID: 20395841.

48. Larsen SG, Pfeffer F, Kørner H. Norwegian Colorectal Cancer Group. Norwegian moratorium on transanal total mesorectal excision. Br J Surg. 2019; 106:1120–1121. PMID: 31304578.

50. Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH, et al. Pathology Review Committee. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002; 26:350–357. PMID: 11859207.
51. Lin HH, Lin JK, Lin CC, Lan YT, Wang HS, Yang SH, et al. Circumferential margin plays an independent impact on the outcome of rectal cancer patients receiving curative total mesorectal excision. Am J Surg. 2013; 206:771–777. PMID: 23871321.

52. McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A. DEPRESsion Screening Data (DEPRESSD) Collaboration. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020; 1. 30. [Epub]. DOI: 10.1177/0962280219889080.
SUPPLEMENTARY MATERIALS
Supplementary Table 1 and Supplementary Fig. 1–3 can be found via
https://doi.org/10.4174/astr.2021.101.3.167
Supplementary Fig. 1
Forest plots of risk ratios of perioperative outcomes. (A) Diverting ileostomy and (B) mortality. A random-effect model was used for meta-analysis of diverting ileostomy. A fixed-effect model was used for meta-analysis of mortality. Risk ratios are shown with 95% confidence interval (CI). TaTME, transanal total mesorectal excision; LaTME, laparoscopic total mesorectal excision; df, degree of freedom.
Supplementary Fig. 2
Forest plots of risk ratios of perioperative outcomes. (A) Ureter or urethral injury, (B) urinary retention, and (C) urinary tract infection. A random-effect model was used for meta-analysis of urinary retention. Fixed-effects models were used for meta-analysis of ureter or urethral injury and urinary tract infection. Risk ratios are shown with 95% confidence interval (CI). TaTME, transanal total mesorectal excision; LaTME, laparoscopic total mesorectal excision; df, degree of freedom.
Supplementary Fig. 3
Funnel plots of risk ratios (RRs) and mean differences of perioperative and pathological outcomes. (A) Conversion rate, (B) circumferential resection margin involvement, (C) major complication (Clavien-Dindo classification III–V), and (D) incompleteness of mesorectum. SE, standard error.
Fig. 1
PRISMA diagram of the search strategy. TaTME, transanal total mesorectal excision; LaTME, laparoscopic total mesorectal excision.
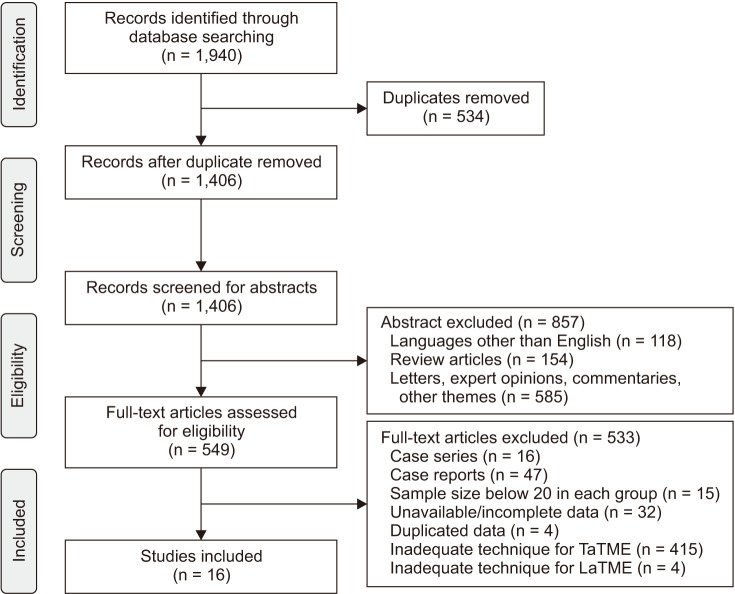
Fig. 2
Forest plots of risk ratios and mean differences of perioperative outcomes. (A) Operative time, (B) intraoperative blood loss and, (C) conversion rate. A random-effect model was used for meta-analysis of operative time. Fixed-effects models were used for meta-analysis of intraoperative blood loss and conversion rate. Mean differences and risk ratios are shown with 95% confidence intervals (CIs). TaTME, transanal total mesorectal excision; LaTME, laparoscopic total mesorectal excision; SD, standard deviation; IV, inverse variance; df, degree of freedom.
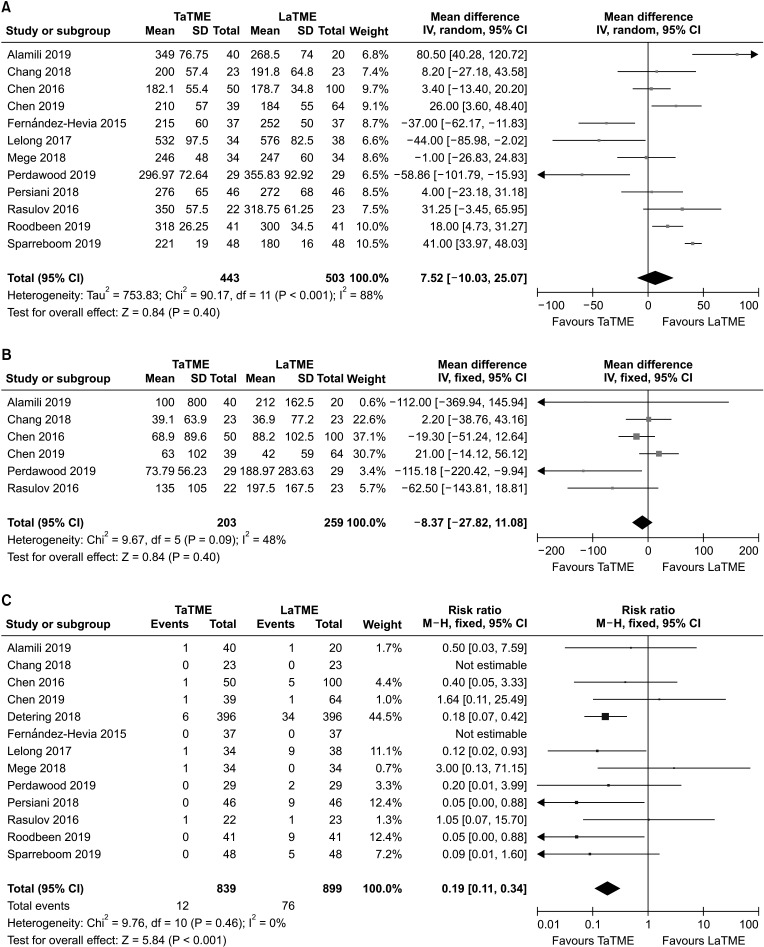
Fig. 3
Forest plots of risk ratios and mean differences of perioperative outcomes. (A) Hospital stay, (B) readmission, and (C) reoperation. A random-effect model was used for meta-analysis of hospital stay. Fixed-effects models were used for meta-analysis of readmission and reoperation. Mean differences and risk ratios are shown with 95% confidence intervals (CIs). TaTME, transanal total mesorectal excision; LaTME, laparoscopic total mesorectal excision; SD, standard deviation; IV, inverse variance; df, degree of freedom.
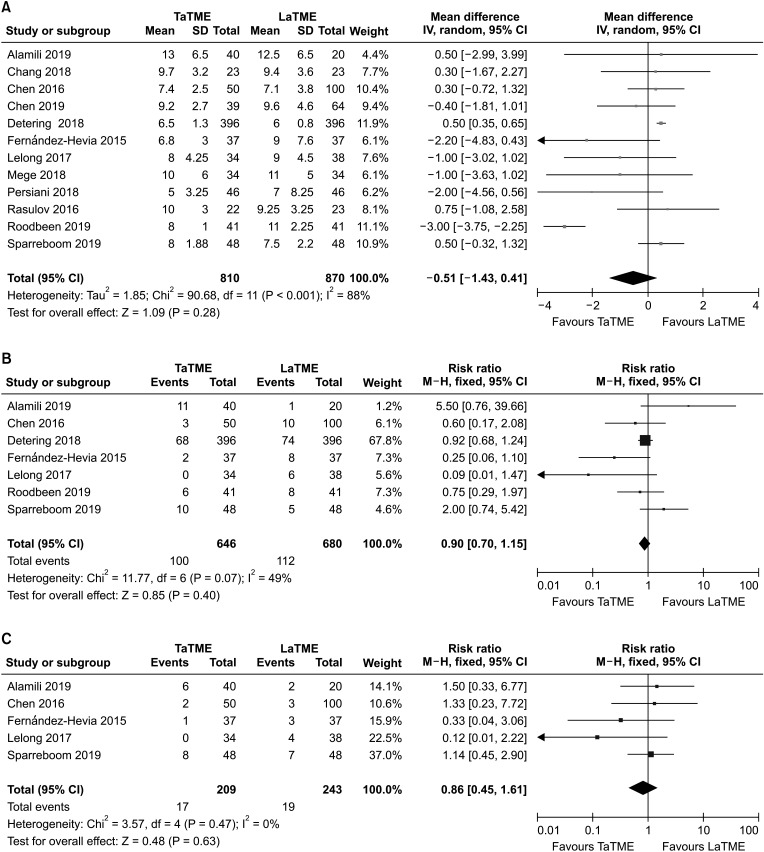
Fig. 4
Forest plots of risk ratios of perioperative outcomes. (A) Major complications (Clavien-Dindo classification III–V), (B) anastomotic leakage, and (C) intestinal obstruction. Fixed-effects models were used for meta-analysis. Risk ratios are shown with 95% confidence intervals (CIs). TaTME, transanal total mesorectal excision; LaTME, laparoscopic total mesorectal excision; df, degree of freedom.
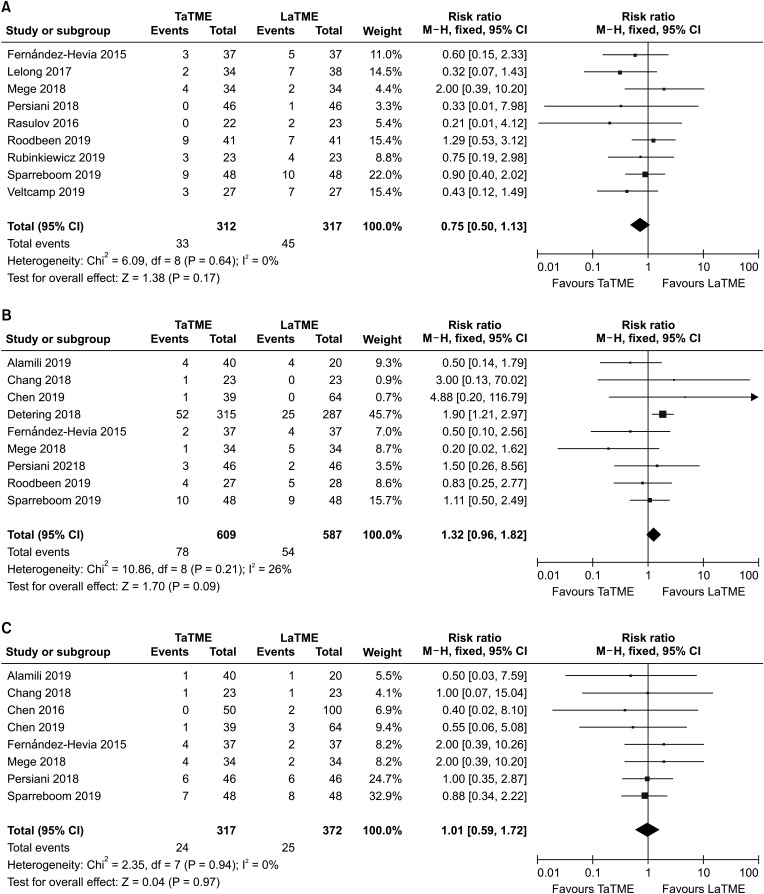
Fig. 5
Forest plots of risk ratios and mean differences of pathological outcomes. (A) Circumferential resection margin involvement, (B) length of circumferential resection margin, (C) distal resection margin involvement, (D) length of distal resection margin, (E) incompleteness of mesorectum, and (F) harvested lymph nodes. Random-effects models were used for meta-analysis of length of circumferential resection margin and distal resection margin. Fixed-effects models were used for meta-analysis of circumferential resection margin and distal resection margin involvement. Mean differences and risk ratios are shown with 95% confidence intervals (CIs). TaTME, transanal total mesorectal excision; LaTME, laparoscopic total mesorectal excision; SD, standard deviation; IV, inverse variance; df, degree of freedom.
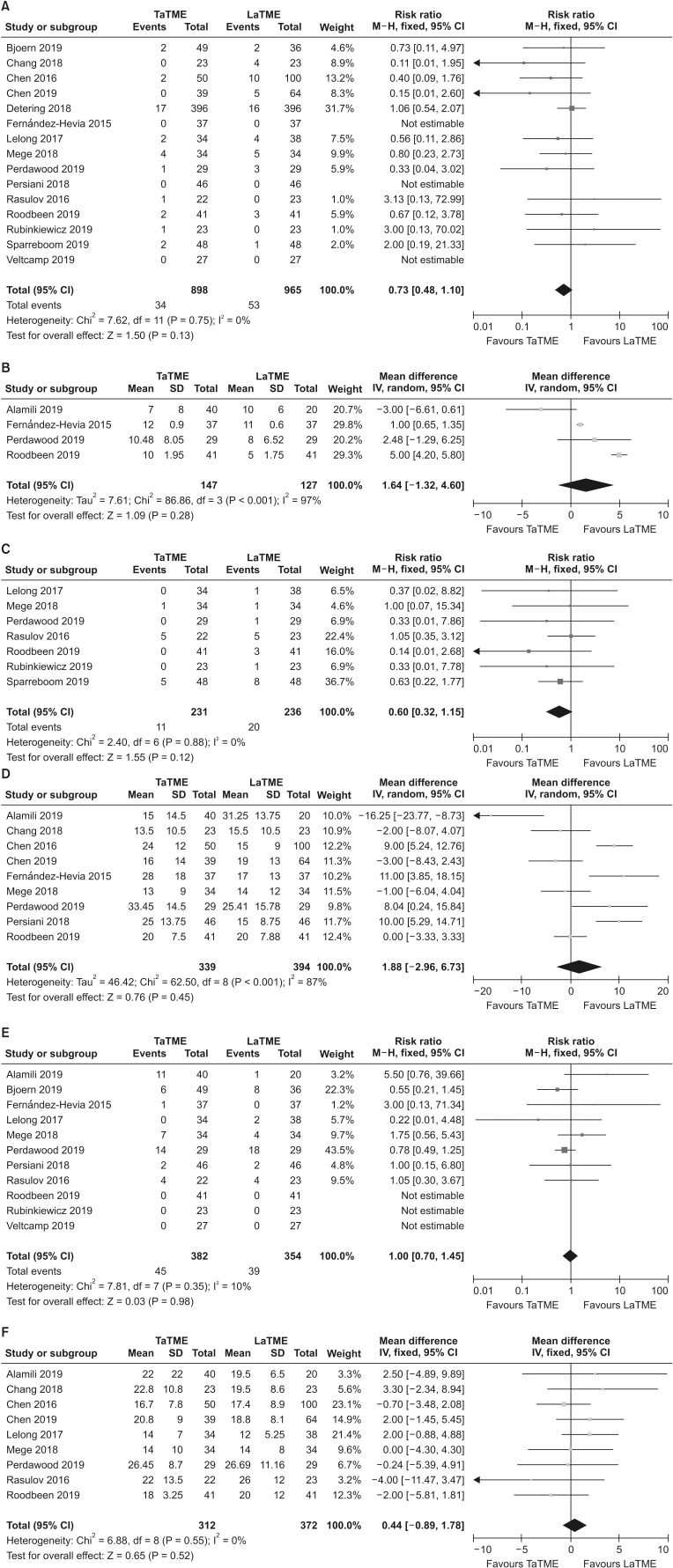
Table 1
Demographics and clinical characteristics of the included studies
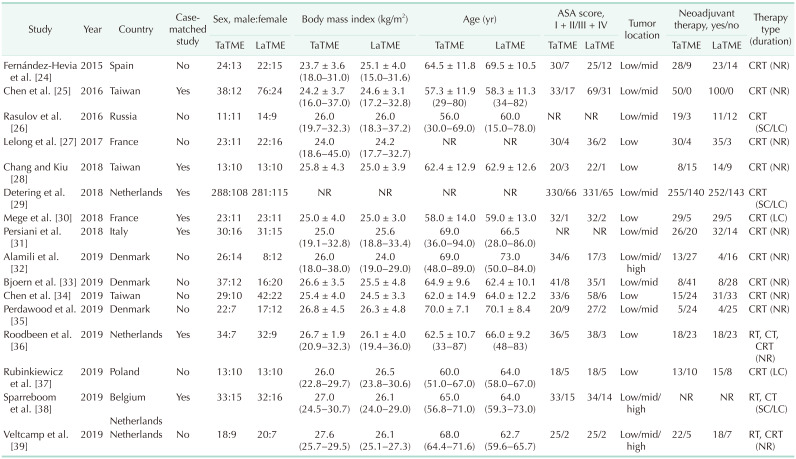
Values are presented as number only, mean ± standard deviation (range), or median (range).
ASA, American society of anesthesiologists; TaTME, transanal total mesorectal excision; LaTME, laparoscopic total mesorectal excision; CRT, chemoradiotherapy; NR, not reported; SC, short-course; LC, long-course; RT, radiotherapy; CT, chemotherapy.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download