This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Preventive measures are needed to reduce the risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among healthcare workers (HCWs). Notably, hospital staff are usually exposed when they are unmasked. There are limited data on the risk of transmission during mealtimes at hospital staff cafeterias. We aimed to evaluate the risk of transmission in cafeterias.
Methods
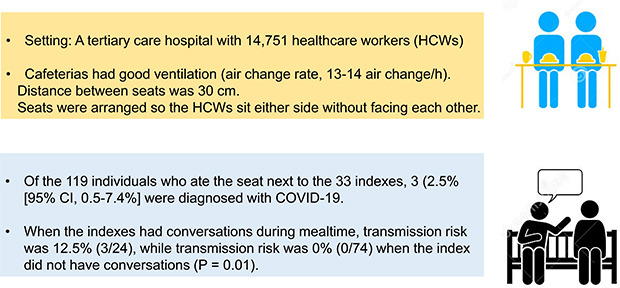
From January 2020 through September 2021, we analyzed the risk of SARS-CoV-2 transmission through closed-circuit television and radio-frequency identification tracking and follow-up testing when 33 HCWs, who were eventually diagnosed as coronavirus disease 2019 (COVID-19), ate in staff cafeterias during the infectious period. The seats were arranged so the HCWs would sit on either side without facing each other. There were no plastic barriers installed, and HCWs were encouraged not to talk during meals.
Results
Three of the 119 individuals who ate at seats next (about 30 cm) to index during the period of transmission and underwent follow-up SARS-CoV-2 polymerase chain reaction tests were diagnosed with COVID-19 (2.5%; 95% confidence interval, 0.5–7.4%). Among the 98 HCWs who were investigated about talking during meals, there was a higher attack rate among those who spoke with each other than among those who did not (12.5% [3/24] vs. 0% [0/74], P = 0.013).
Conclusion
The risk of transmission in a hospital’s employee cafeterias is not high with side-by-side seating, especially in the absence of conversation.
Keywords: SARS-CoV-2, COVID-19, Transmission, Meal, Healthcare Workers
INTRODUCTION
Preventive measures are needed to reduce the risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among healthcare workers (HCWs) due to the risk of transmission to vulnerable patients, HCW restrictions, and staff shortages due to exposure.
12 Hospital staff are usually exposed when they are unmasked. However, mask use is impossible during mealtimes, and physical distancing cannot be maintained in many staff cafeterias that were designed before the pandemic.
34 Therefore, many hospitals are implementing preventive measures, such as improving ventilation, discouraging talking, installing plastic barriers, and implementing social distancing in cafeterias. However, there are limited data on the risk of transmission during mealtimes at hospital staff cafeterias. Therefore, we aimed to evaluate the risk of transmission in the cafeterias of a tertiary care hospital in Seoul, South Korea.
METHODS
This study was performed at the Asan Medical Center, a 2,700-bed tertiary care hospital. There are 14,751 HCWs (all employees, students, and researchers). HCWs daily self-monitored their symptoms and performed SARS-CoV-2 testing at our hospital when they showed symptoms or had a known exposure. Whenever a case of coronavirus disease 2019 (COVID-19) was detected, we performed contact tracing, as previously reported.
5 From January 2020 through September 2021, we analyzed the risk of COVID-19 transmission, in hospital staff cafeterias, to staff members seated close to individuals who would later test positive for SARS-CoV-2 infection (index). We reviewed closed-circuit television (CCTV) footage and radio-frequency identification (RFID) tagging in the cafeterias to identify contacts. We tracked all of the index’s movements with CCTV footage. All individuals sitting next to index were traced, and the relevant employee information was acquired through the time information from RFID tagging. We did not check individuals sitting right next to the index’s empty seats upon leaving the table after eating. We notified individuals who were exposed inside or outside the cafeterias, and they underwent testing for SARS-CoV-2. As previously reported,
56 we also performed contact tracing outside the cafeteria. The exposure to the COVID-19 patients included close and non-close contacts, which were defined as previously reported.
6 Briefly, close contacts were defined as: 1) those who were in close proximity (< 6 feet) for at least cumulative 15 minutes from 2 days before the symptom development in the index, or from 2 days before the date of collection of a positive specimen in indexes that were asymptomatic, 2) individuals who had a meal with the index, or 3) contact with the index patient when an aerosol-generating event that occurred without appropriate personal protective equipment (PPE). Non-close contacts were defined as those who did not meet the criteria of close contact but had possible temporal or spatial contact with the confirmed patient.
There were three cafeterias for HCWs, measuring 980 m2, 829 m2, and 786 m2. All three cafeterias were well ventilated, with a central heating, ventilation, and air conditioning (HVAC) system in place (air change rate, 13–14 air change/h). Everyone served their own food on their plates before eating. The distance between seats was about 30 cm, and the seats were arranged so the employees would sit only on either side of one another without facing each other. Employees were encouraged not to talk during their meals. There was no plastic barrier installed. Since there were many employees, it was not possible for them to sit far enough apart from each other to adhere to social distancing guidelines during most of the mealtimes evaluated. We advised staff members to leave an empty seat between cafeteria patrons whenever possible, and not all index ate only during the busiest lunch hour. Some index did not have other staff sitting next to them because they ate breakfast or dinner when cafeterias were less crowded.
From March 5, 2021, vaccinations for HCWs began at the COVID-19 vaccine center at our hospital. Four COVID-19 vaccines have were used at our hospital during the study period: ChAdOx1 nCoV-19 (AstraZeneca, Cambridge, UK), BNT162b2 (Pfizer, New York, NY, USA), mRNA-1273 (Moderna, Cambridge, MA, USA), and heterologous vaccination with ChAdOx1 nCoV-19 followed by BNT162b2. Two doses of ChAdOx1 nCoV-19 were administered 12 weeks apart, BNT162b2 doses were administered 3 weeks apart, and mRNA-1273 doses were administered 4 weeks apart. Heterologous vaccination was administered using a 12-week interval.
Categorical variables were analyzed using the χ2 test or Fisher’s exact test, as appropriate. Statistical analyses were performed using SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, NY, USA), and P values < 0.05 were considered statistically significant.
Ethics statement
This study was approved by the Institutional Review Board (IRB) of Asan Medical Center, which waived the requirement for written informed consent (IRB No. 2021-0024).
RESULTS
From January 2020 through September 2021, 77 HCWs were diagnosed with COVID-19. Of these, 44 were excluded from this study because they were diagnosed during the quarantine period, or they did not have a meal at any employee cafeteria at the hospital. Therefore, we analyzed the cafeteria contacts of 33 HCWs with COVID-19. Of the 33 index, 27 (82%) were symptomatic at diagnosis, and nine (27%) were vaccinated (six were fully vaccinated). The median cycle threshold (Ct) value of 30 HCWs whose Ct values were available at diagnosis was 18.56 (interquartile range [IQR], 15.33–22.48). The remaining three index underwent SARS-CoV-2 testing outside our hospital, and the detailed diagnostic data were not available to us.
There were 119 individuals who ate at seats next to index. Of these, 38 (32%) were vaccinated (26 were fully vaccinated, and 12 were partially vaccinated). Three of these individuals were diagnosed with COVID-19 (2.5%; 95% confidence interval [CI], 0.5–7.4%) (
Table 1). One of the individuals diagnosed with COVID-19 was fully vaccinated. All three were close colleagues with an index, so they had other exposures, such as shared coffee breaks in addition to mealtimes (
Table 2). Therefore, we analyzed the risk of transmission to contacts according to their relationships with index. Among 45 individuals who did not know any index patient, none was diagnosed with COVID-19 even though some sat next to index while eating (0%; 95% CI, 0–8.2%). The cumulative exposure times during the mealtimes of infected individuals were 86 (four meals consumed during the infectious period), 10 (one meal), and 21 (one meal) minutes, respectively. The median cumulative exposure time of 103 non-infected exposed individuals, whose exposure times were recorded, was 12 minutes (IQR, 10–15) (
P = 0.157).
Table 1
Risk of SARS-CoV-2 transmission when dining next to an index in an employee cafeteria

|
Transmission factors |
Values |
P value |
|
Overall transmission risk |
2.5% (3/119) |
|
|
Transmission risk according to conversation or vaccination |
|
|
|
Index and contact acquainted |
4.1% (3/74) |
0.289 |
|
Index and contact not acquainted |
0% (0/45) |
|
Conversing with one anothera
|
12.5% (3/24) |
0.013 |
|
No conversationa
|
0% (0/74) |
|
Vaccinated indexb
|
2.9% (1/34) |
> 0.999 |
|
Unvaccinated index |
2.4% (2/85) |
|
Vaccinated contactc
|
2.6% (1/38) |
> 0.999 |
|
Unvaccinated contact |
2.5% (2/81) |

Table 2
Characteristics of the index who transmitted SARS-CoV-2
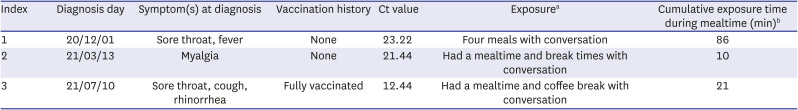
|
Index |
Diagnosis day |
Symptom(s) at diagnosis |
Vaccination history |
Ct value |
Exposurea
|
Cumulative exposure time during mealtime (min)b
|
|
1 |
20/12/01 |
Sore throat, fever |
None |
23.22 |
Four meals with conversation |
86 |
|
2 |
21/03/13 |
Myalgia |
None |
21.44 |
Had a mealtime and break times with conversation |
10 |
|
3 |
21/07/10 |
Sore throat, cough, rhinorrhea |
Fully vaccinated |
12.44 |
Had a mealtime and coffee break with conversation |
21 |

During the early period of the pandemic, we only used CCTV and RFID tracking to determine which individuals sat next to index. Consequently, for 21 of the 119 HCWs who ate at seats next to index, there was no information captured about whether these HCWs had conversations with index during cafeteria mealtimes. For the remaining 98 HCWs (82%), CCTV investigations included determining if they had conversations during cafeteria mealtimes. When the index had conversations during mealtimes, the transmission risk was 12.5% (3/24); when the index did not have conversations, the transmission risk was 0% (0/74,
P = 0.013). There were no significant differences in transmission risk according to index patient vaccination status (vaccinated 2.9% vs. unvaccinated 2.4%,
P > 0.999) or contact vaccination status (vaccinated 2.6% vs. unvaccinated 2.5%,
P > 0.999) (
Table 1).
DISCUSSION
We found that the overall transmission rate was 2.5% in the hospital staff cafeterias, with side-by-side seating (with about 30-cm spacing) and good ventilation. When index had conversations during mealtimes, the transmission risk was significantly higher than when index remained silent (12.5% vs. 0%, P = 0.013). These findings provide important information for policy decisions regarding employee or student cafeterias.
Indoor dining poses a risk of SARS-CoV-2 transmission as individuals are unmasked and have frequent conversations at mealtime, permitting the emission of respiratory droplets and aerosols. The United States Centers for Disease Control and Prevention evaluated the association between allowing on-site restaurant dining and COVID-19 cases and deaths between March and December 2020.
7 Increases in COVID-19 cases and deaths were significantly associated with indoor dining at restaurants after on-premises restaurant dining had been permitted for > 40 days.
7 In our study, we identified three cases of SARS CoV-2 transmission, and all three index were close colleagues with the contacts who acquired the infections; moreover, all infected contacts had conversations with index during cafeteria mealtimes. It is possible that transmission occurred at other places in these three cases, but our data indicate that transmission did not occur in 74 individuals in the absence of mealtime conversation and physical distancing. Asadi et al.
8 performed experiments using an aerodynamic particle sizer, and they demonstrated that the number and concentration of particles increased in association with speaking compared with breathing alone. These findings support the importance of educating employees to refrain from conversation during meals.
Installation of physical barriers is suggested, particularly in restaurants, wherein maintaining physical distances of at least 6 feet is difficult.
9 However, barriers could change the airflow in a room and lead to disruption of normal ventilation.
10 Therefore, viruses could accumulate in non-ventilated areas. Our hospital did not install plastic barriers between seats, but the risk of transmission was low. Further research is needed to ascertain whether plastic barriers in the cafeteria are useful for preventing transmission or if they are associated with increased transmission.
Our study had some limitations. First, we only used CCTV and RFID to trace individuals who sat next to index because tracing is labor intensive. We may have missed more distant transmissions. However, we thoroughly investigated the contact tracing and determined if there were overlapping movement between infected HCWs. Additionally, we immediately informed all HCWs when the index ate meals in the employee cafeterias and encouraged SARS-CoV-2 polymerase chain reaction (PCR) testing for any concerns about the overlapping movements. Therefore, we believe that missed cases during mealtime in the employee cafeterias during the study period were unlikely. As shown in
Supplementary Fig. 1, our hospital has done well to minimize nosocomial SARS-CoV-2 transmission. Second, the number of secondary cases may seem low considering the large number of employees in our hospital. However, we extensively traced the individuals who sat next to index and performed PCR testing. We believe that the transmission rate could have been kept low thanks to several preventive measures. Additionally, some may argue that secondary cases in the study could have acquired infection from other individuals rather than from the presumed index cases because we did not perform whole-genome sequencing (WGS) to confirm infection sources. However, as shown in our previous study,
5 WGS was not helpful for identifying infection sources when there were no epidemiologic links. Indeed, we did not find any epidemiologic links other than contact with index in our thorough re-investigation. Therefore, we believe that SARS-CoV-2 exposure in places other than the staff cafeterias in these three HCWs is unlikely considering the extensive epidemiologic investigations performed by our infection control team with the help of government epidemiologic investigators and the relatively low community transmission level during the study period in South Korea (low or moderate transmission: < 10 or 10–49 new cases per 100,000 persons in the week).
5 Third, transmission risk did not differ according to levels of acquaintance between index and contacts. This may have been due to the small sample size. Further larger-scale research is warranted to validate our findings. Fourth, the finding that the risk of transmission was higher when index had conversations may have resulted from differences in exposure times rather than conversation per se. Fifth, this was a small, single-center study in Korea where the community transmission level was low or moderate. Notably, however, due to the relatively low level of community transmission, we could more thoroughly trace contacts and rule out other sources of SARS-CoV-2 acquisition. However, vaccine effectiveness against SARS-CoV-2 transmission during mealtime could not be demonstrated by this analysis because of the lack of study power. Finally, the cafeterias were crowded during peak hours, and physical distancing could not be maintained. Therefore, a large multicenter study including cafeterias with different characteristics is warranted to validate our findings.
In conclusion, the risk of transmission in well-ventilated (air change rate, 13–14 air change/h) employee cafeterias was low with side-by-side seating, especially in the absence of conversation. Seating arrangement considerations, maintaining ventilation, and refraining from conversation during meals are important strategies for preventing SARS-CoV-2 transmission.