Abstract
Purpose
To investigate the effects of dose rate on intensity-modulated radiation therapy (IMRT) quality assurance (QA).
Methods
We performed gamma tests using portal dose image prediction and log files of a multileaf collimator. Thirty treatment plans were randomly selected for the IMRT QA plan, and three verification plans for each treatment plan were generated with different dose rates (200, 400, and 600 monitor units [MU]/min). These verification plans were delivered to an electronic portal imager attached to a Varian medical linear accelerator, which recorded and compared with the planned dose. Root-mean-square (RMS) error values of the log files were also compared.
Results
With an increase in dose rate, the 2%/2-mm gamma passing rate decreased from 90.9% to 85.5%, indicating that a higher dose rate was associated with lower radiation delivery accuracy. Accordingly, the average RMS error value increased from 0.0170 to 0.0381 cm as dose rate increased. In contrast, the radiation delivery time reduced from 3.83 to 1.49 minutes as the dose rate increased from 200 to 600 MU/min.
Radiation therapy is a core component of cancer treatment considering that >50% of patients receive radiation therapy during the course of their treatment, and this percentage is bound to continually increase in the future [1,2]. The goal of this therapy is to deliver maximal radiation dose to tumors while reducing the dose to normal tissues. Intensity-modulated radiation therapy (IMRT) manipulates radiation beams to conform to the shape of a tumor, thereby avoiding damage to healthy tissues [3,4]. As IMRT is associated with a lower incidence of radiation toxicity, more patients are currently being treated with IMRT. However, IMRT requires the complex movement of a multileaf collimator (MLC) to modulate the intensity of beams from a medical linear accelerator (linac). For several multifaceted reasons, IMRT quality assurance (QA) is pivotal for the verification of treatment plans and beam delivery. Many factors, such as MLC leaf position, MLC circuit system, and detector, potentially contribute to suboptimal IMRT QA.
For beam intensity modulation, the step-and-shoot (segmental) MLC (SMLC) and dynamic MLC (DMLC) approaches are widely used. The SMLC technique uses MLC to make multiple segments and delivers radiation while the MLC is fixed; the radiation beam is off when the MLC is moving. In contrast, in the DMLC technique, the MLC keeps moving, and radiation is continuously delivered.
MLC movement is controlled by linac and MLC controllers. The total beam is controlled by an ion chamber located in the linac head, and the segment dose and leaf position are controlled by the MLC controller. Both the SMLC and DMLC methods show undershoot/overshoot effects. The C-series linac model used by us communicates with the MLC controller every 50 ms. Because of a delay in communication, the control is delayed by approximately 50 to 80 ms, and overshoot occurs. When the total beam system reaches the planned dose, the beam is turned off, and the last segment undershoots. The overshoot is challenging to detect in dynamic sliding window IMRT, but it does exist and is reported during the first and last 65 ms [5,6].
MLC movement is a major source of error between treatment planning and delivery [7,8]. The dose rate is needed to correctly synchronize the MLC position. A higher dose rate is beneficial to patients because of their comfort and less intrafraction motion [9]. However, higher dose rates can lead to inaccurate radiation delivery due to MLC positional error or the overshoot/undershoot phenomenon [10].
Herein, our main objective was to evaluate the impact of radiation dose rate on the accuracy of dynamic IMRT dose delivery, as measured using portal dose image prediction (PDIP) and log files of the MLC with different dose rates (200, 400, and 600 monitor units [MU]/min) for lung, prostate, and head and neck (H&N) cancer patient groups.
Thirty (10 lung, 10 prostate, and 10 H&N cancer) patients who had received radiation therapy were randomly selected. Patient treatment comprised treatment planning, pretreatment delivery with verification, and actual treatment (Fig. 1). Three verification plans were created using the Eclipse treatment planning system v8.9 (Varian Medical Systems, Palo Alto, CA, USA) for each patient with different dose rates (200, 400, and 600 MU/min) and diverse MLC speeds. Measurements were performed with an aSi1000 amorphous silicon electronic portal imaging device attached to a linac (Varian Clinac iX; Varian Medical Systems). The energy was 6 MV for lung (six fields) and H&N (seven fields) and 10 MV for prostate (seven fields) cancer. The prescription was 200 cGy. The mean MU for the 30-patient verification plan was 200, 400, and 600 MU/min at 821, 961, and 1,106 MU, respectively. To avoid mechanical uncertainties, the gantry angle was fixed to 0° and the source-to-imager distance to 100 cm.
The effects of dose rate on patients with cancer were evaluated with regard to the following aspects: 1) Gamma index analysis by PDIP, 2) MLC positional error by DMLC log (DynaLog) file, and 3) Beam-on time difference.
The PDIP (Portal Dosimetry v8.8; Varian Medical Systems) algorithm calculates the predicted radiation fluency to the electronic portal imaging device and compares it with the measurement [11]. We used the gamma-index method using the criteria of 3%/3-mm and 2%/2-mm to evaluate the comparison [12]. The gamma test provides a pass–fail criterion, and two distributions are considered in agreement when the gamma value is <1. The gamma passing rate of >95% is widely considered to be clinically acceptable.
A DynaLog file is a record of MLC delivery at every 50 ms. A vendor-provided software (Dynalog File Viewer v7.0; Varian Medical Systems) calculates root-mean-square (RMS) errors between planned and actual leaf positions. RMS errors were recorded for each of the aforementioned verification plans.
The vendor-provided software also generates a chart history, which provides the actual beam-on time during radiation therapy. We recorded the average treatment time of 30 patients QA and assumed that other time difference such as set-up or gantry rotation is negligible between three different dose rates.
Overall, increased dose rates were associated with a decrease in treatment time but an increase in radiation delivery uncertainties. ANOVA was used as a post-hoc test (SAS v9.4; SAS Institute, Cary, NC, USA). RMS errors and beam-on time were statistically significant (P<0.05).
For all disease sites, the average gamma passing rate achieved with the 3%/3-mm (Fig. 2) and 2%/2-mm criteria (Fig. 3) was 97.7% and 88.0%, respectively. The passing rate achieved with the 3%/3-mm criterion decreased from 98.4% to 97.9% to 96.9% with 200, 400, and 600 MU/min, respectively, indicating that radiation delivery accuracy decreased with an increase in dose rates. The impact of dose rates was more distinguishable in the case of patients with H&N cancer because their treatment plans were relatively complicated; further, the treatment plans showed large variations (i.e., higher standard deviation) within the patient group.
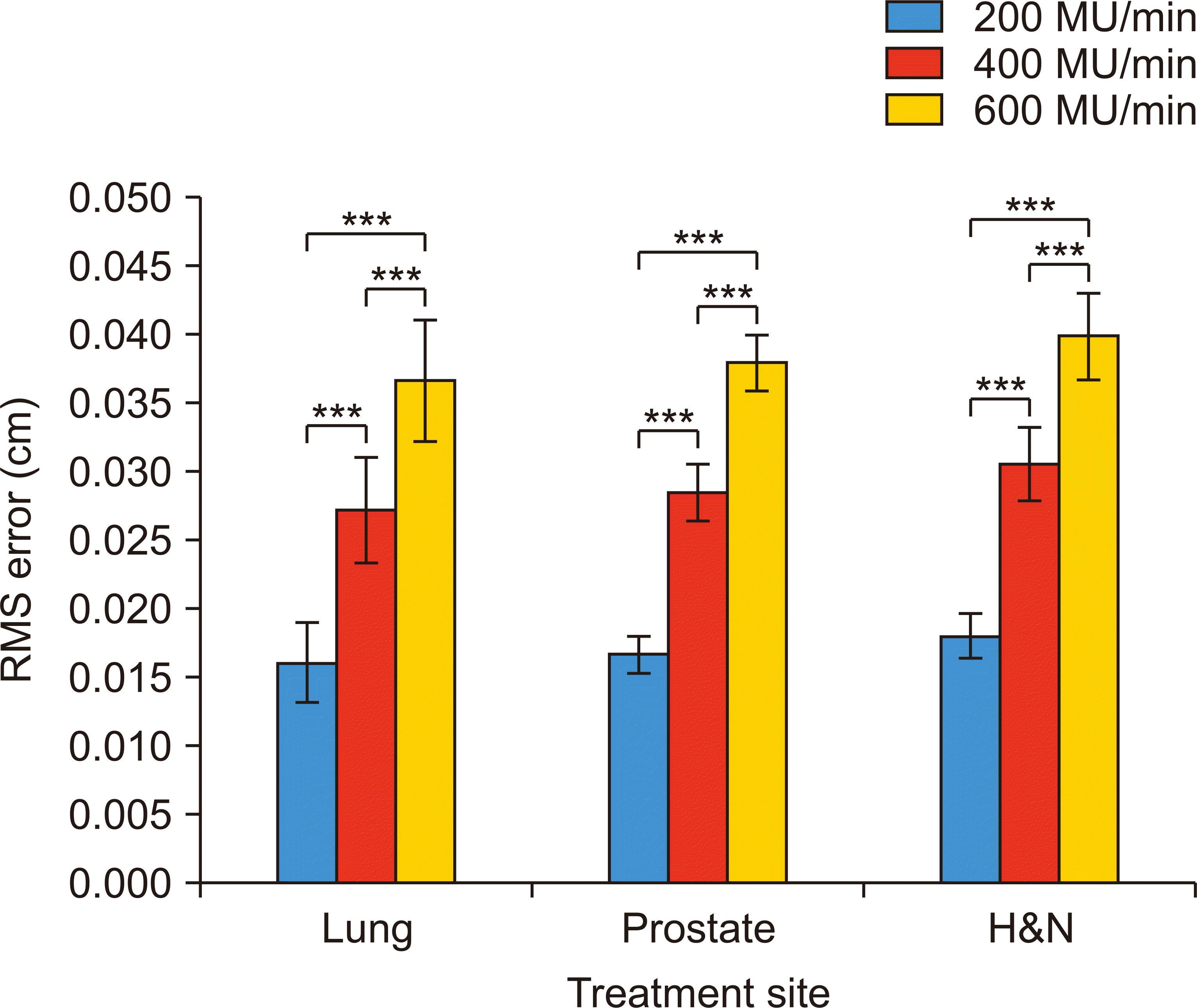
The increased leaf-speed also increased the MLC positional RMS error (Fig. 4). The average RMS errors were 0.0170, 0.0287, and 0.0381 cm with 200, 400, and 600 MU/min, respectively. These values met the criterion according to the American Association of Physicists in Medicine Task Group-142 [13]. With regard to disease sites, H&N cancer treatment plans showed the largest errors, whereas lung cancer treatment plans showed the smallest ones.
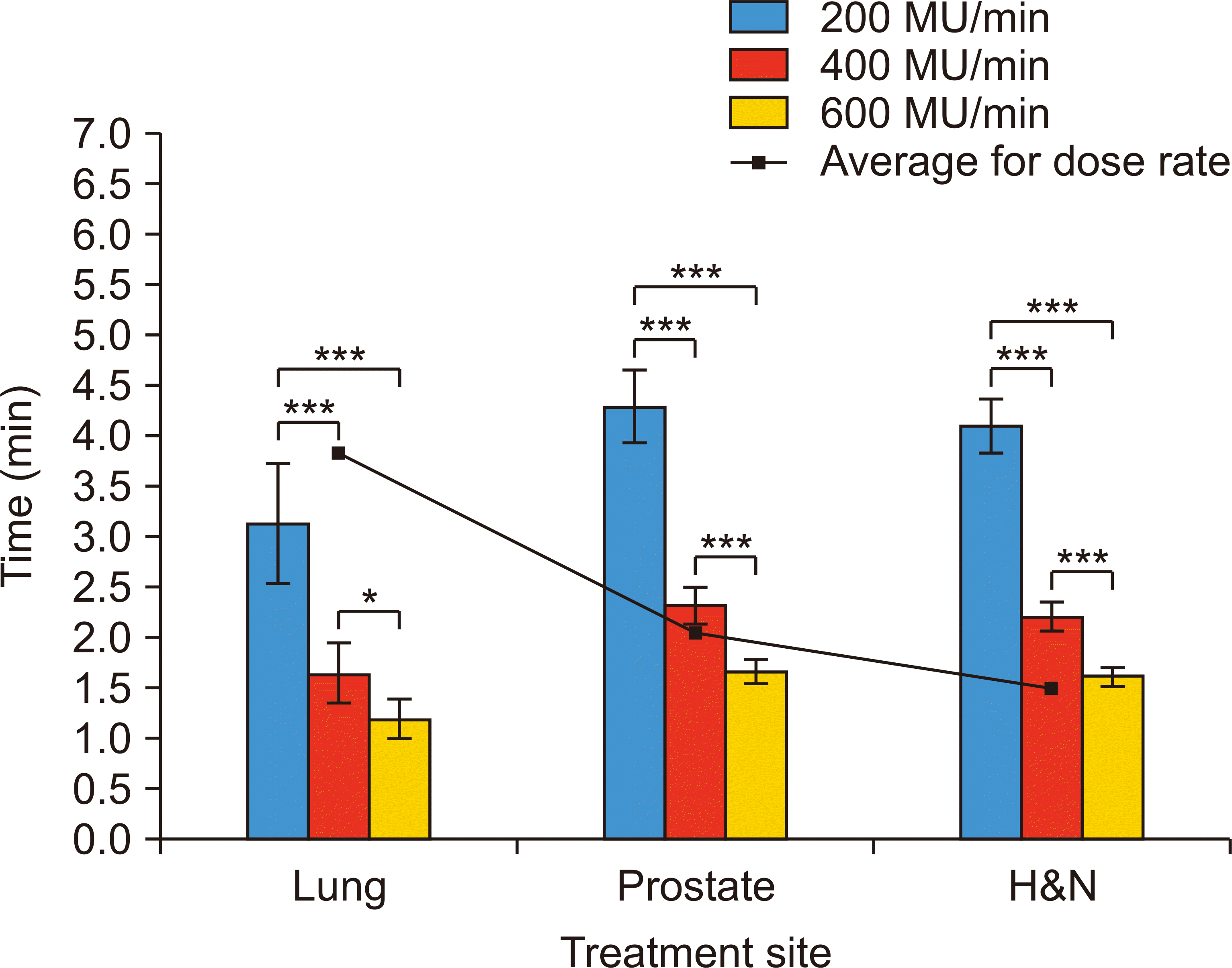
As the dose rate increased, the treatment time became shorter (Fig. 5); however, this decrease was not linearly proportional to dose rates, because an increase in MU was also observed. If the dose rate increases, the leaf motion calculator algorithm creates more fluency segments, consequently increasing total MU. The average numbers of segments were 101, 116, and 129 with 200, 400, and 600 MU/min, respectively. The corresponding average MU/segments were 1.13, 1.08, and 1.07 with 200, 400, and 600 MU/min, respectively. An increase in the dose rate from 200 to 600 MU/min reduced beam-on time by up to 61.1%.
Radiation dose validation is a key step in radiation therapy, as recommended by many international organizations, including the American Association of Physicists in Medicine and American Society for Radiation Oncology. Various QA techniques have been developed, involving the use of, for example, ionization chambers, radiographic films, diode arrays, and ionization chamber arrays; however, such systems involve additional costs and efforts. On the other hand, portal dosimetry is easy considering that an imaging device is already attached to a linac to verify patient positioning [14]. MLC position is also recorded in the recent linac, enabling the determination of MLC positional accuracy [15]. Therefore, we herein used the portal dosimetry and DynaLog RMS error method to investigate the impact of dose rates on radiation delivery accuracy.
As previously reported, the average RMS error associated with the SMLC and DMLC techniques is 0.0008 and 0.032 cm, respectively, and these values are similar to our results. RMS errors linearly increase with dose rates and leaf speed. Leaf speed is crucial in DMLC, and MLC performance can be evidently improved by limiting leaf speed [16]. In a study investigating MLC positional errors, random errors were found to not affect dosimetry. However, systematic errors may occur due to communication delay, which may have a dosimetric effect; thus, strict tolerance is required [17].
Increased dose rates have recently received much attention from radiation treatment society. A reduction in treatment time when dose rates are higher is an attractive alternative as patient comfort is more and intrafractional motion is less. Moreover, higher dose rates can effectively reduce the fraction of blood irradiated to low doses, which can avoid radiation-induced lymphopenia [18,19]. In addition, extremely high dose rates, known as FLASH, reportedly show normal tissue sparing effect compared to conventional dose rates [20]. However, as observed in this study, dose rates should be carefully selected in a clinical setting because they can reduce radiation delivery accuracy.
Our findings revealed that increased dose rates reduced radiation delivery accuracy as well as minimized treatment time. Dose rates of up to 600 MU/min showed clinically acceptable delivery accuracy. We believe that multi-institutional studies can provide a roadmap to determine the highest dose rate with optimal delivery accuracy in clinical settings.
Acknowledgements
This work was supported by grants from the National Research Foundation of Korea (NRF, No. 2021R1C1C1005930 and No. 2018R1A2B2005343) funded by the Korea government (MSIT).
Notes
Availability of Data and Materials
All relevant data are within the paper and its Supporting Information files.
Author Contributions
Conceptualization: Kwon Hee Kim, Tae Seong Back, and Wonmo Sung. Data curation and formal analysis: Kwon Hee Kim, Tae Seong Back, and Eun Ji Chung. Investigation: Kwon Hee Kim, Tae Seong Back, and Eun Ji Chung. Methodology: Kwon Hee Kim, Tae Suk Suh, and Wonmo Sung. Supervision: Tae Suk Suh and Wonmo Sung. Validation: Kwon Hee Kim, Tae Seong Back, and Eun Ji Chung. Writing-original draft: Kwon Hee Kim. Writing-review & editing: Tae Suk Suh and Wonmo Sung.
References
1. Baskar R, Lee KA, Yeo R, Yeoh KW. 2012; Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 9:193–199. DOI: 10.7150/ijms.3635. PMID: 22408567. PMCID: PMC3298009.

2. Baumann M, Ebert N, Kurth I, Bacchus C, Overgaard J. 2020; What will radiation oncology look like in 2050? A look at a changing professional landscape in Europe and beyond. Mol Oncol. 14:1577–1585. DOI: 10.1002/1878-0261.12731. PMID: 32463984. PMCID: PMC7332208.

3. Webb S. 2015. Intensity-modulated radiation therapy. CRC Press;Boca Raton: DOI: 10.1201/9781420034110.
4. Bortfeld T. 2006; IMRT: a review and preview. Phys Med Biol. 51:R363–R379. DOI: 10.1088/0031-9155/51/13/R21. PMID: 16790913.

5. Ezzell GA, Chungbin S. 2001; The overshoot phenomenon in step-and-shoot IMRT delivery. J Appl Clin Med Phys. 2:138–148. DOI: 10.1120/jacmp.v2i3.2607. PMID: 11602010. PMCID: PMC5726041.

6. Yu CY, Wan SW, Weng YC, Hsu CH. 2021; Three corrections for overshoot effect improved the dose for step-and-shoot intensity-modulated radiation therapy. PLoS One. 16:e0250243. DOI: 10.1371/journal.pone.0250243. PMID: 33891639. PMCID: PMC8064569.

7. Li J, Wiersma RD, Stepaniak CJ, Farrey KJ, Al-Hallaq HA. 2012; Improvements in dose accuracy delivered with static-MLC IMRT on an integrated linear accelerator control system. Med Phys. 39:2456–2462. DOI: 10.1118/1.3701778. PMID: 22559616.

8. Xia P, Chuang CF, Verhey LJ. 2002; Communication and sampling rate limitations in IMRT delivery with a dynamic multileaf collimator system. Med Phys. 29:412–423. DOI: 10.1118/1.1449496. PMID: 11929023.

9. Langen KM, Willoughby TR, Meeks SL, Santhanam A, Cunningham A, Levine L, et al. 2008; Observations on real-time prostate gland motion using electromagnetic tracking. Int J Radiat Oncol Biol Phys. 71:1084–1090. DOI: 10.1016/j.ijrobp.2007.11.054. PMID: 18280057.

10. Njeh CF, Salmon HW, Schiller C. 2017; The impact of dose rate on the accuracy of step-and-shoot intensity-modulated radiation therapy quality assurance using varian 2300CD. J Med Phys. 42:206–212. DOI: 10.4103/jmp.JMP_18_17. PMID: 29296034. PMCID: PMC5744448.

11. Bresciani S, Poli M, Miranti A, Maggio A, Di Dia A, Bracco C, et al. 2018; Comparison of two different EPID-based solutions performing pretreatment quality assurance: 2D portal dosimetry versus 3D forward projection method. Phys Med. 52:65–71. DOI: 10.1016/j.ejmp.2018.06.005. PMID: 30139611.

12. Low DA, Dempsey JF. 2003; Evaluation of the gamma dose distribution comparison method. Med Phys. 30:2455–2464. DOI: 10.1118/1.1598711. PMID: 14528967.

13. Klein EE, Hanley J, Bayouth J, Yin FF, Simon W, Dresser S, et al. 2009; Task Group 142 report: quality assurance of medical accelerators. Med Phys. 36:4197–4212. DOI: 10.1118/1.3190392. PMID: 19810494.
14. van Elmpt W, McDermott L, Nijsten S, Wendling M, Lambin P, Mijnheer B. 2008; A literature review of electronic portal imaging for radiotherapy dosimetry. Radiother Oncol. 88:289–309. DOI: 10.1016/j.radonc.2008.07.008. PMID: 18706727.

15. Stell AM, Li JG, Zeidan OA, Dempsey JF. 2004; An extensive log-file analysis of step-and-shoot intensity modulated radiation therapy segment delivery errors. Med Phys. 31:1593–1602. DOI: 10.1118/1.1751011. PMID: 15259664.

16. Kerns JR, Childress N, Kry SF. 2014; A multi-institution evaluation of MLC log files and performance in IMRT delivery. Radiat Oncol. 9:176. DOI: 10.1186/1748-717X-9-176. PMID: 25112533. PMCID: PMC4251954.

17. Rangel A, Dunscombe P. 2009; Tolerances on MLC leaf position accuracy for IMRT delivery with a dynamic MLC. Med Phys. 36:3304–3309. DOI: 10.1118/1.3134244. PMID: 19673226.

18. Shin J, Xing S, McCullum L, Hammi A, Pursley J, Correa CA, et al. 2021; HEDOS-a computational tool to assess radiation dose to circulating blood cells during external beam radiotherapy based on whole-body blood flow simulations. Phys Med Biol. 66:164001. DOI: 10.1088/1361-6560/ac16ea. PMID: 34293735.
19. Hammi A, Paganetti H, Grassberger C. 2020; 4D blood flow model for dose calculation to circulating blood and lymphocytes. Phys Med Biol. 65:055008. DOI: 10.1088/1361-6560/ab6c41. PMID: 32119649. PMCID: PMC8268045.

20. Jin JY, Gu A, Wang W, Oleinick NL, Machtay M, Spring Kong FM. 2020; Ultra-high dose rate effect on circulating immune cells: a potential mechanism for FLASH effect? Radiother Oncol. 149:55–62. DOI: 10.1016/j.radonc.2020.04.054. PMID: 32387486. PMCID: PMC7442672.

Fig. 1
Quality assurance (QA) procedure for intensity-modulated radiation therapy. PDIP, portal dose image prediction; EPID, electronic portal imaging device; 2D, two-dimensional; 3D, three-dimensional.

Fig. 2
Gamma passing rate with the 3%/3-mm criterion as a function of dose rates for lung, prostate, and head and neck (H&N) disease sites (*P<0.05).
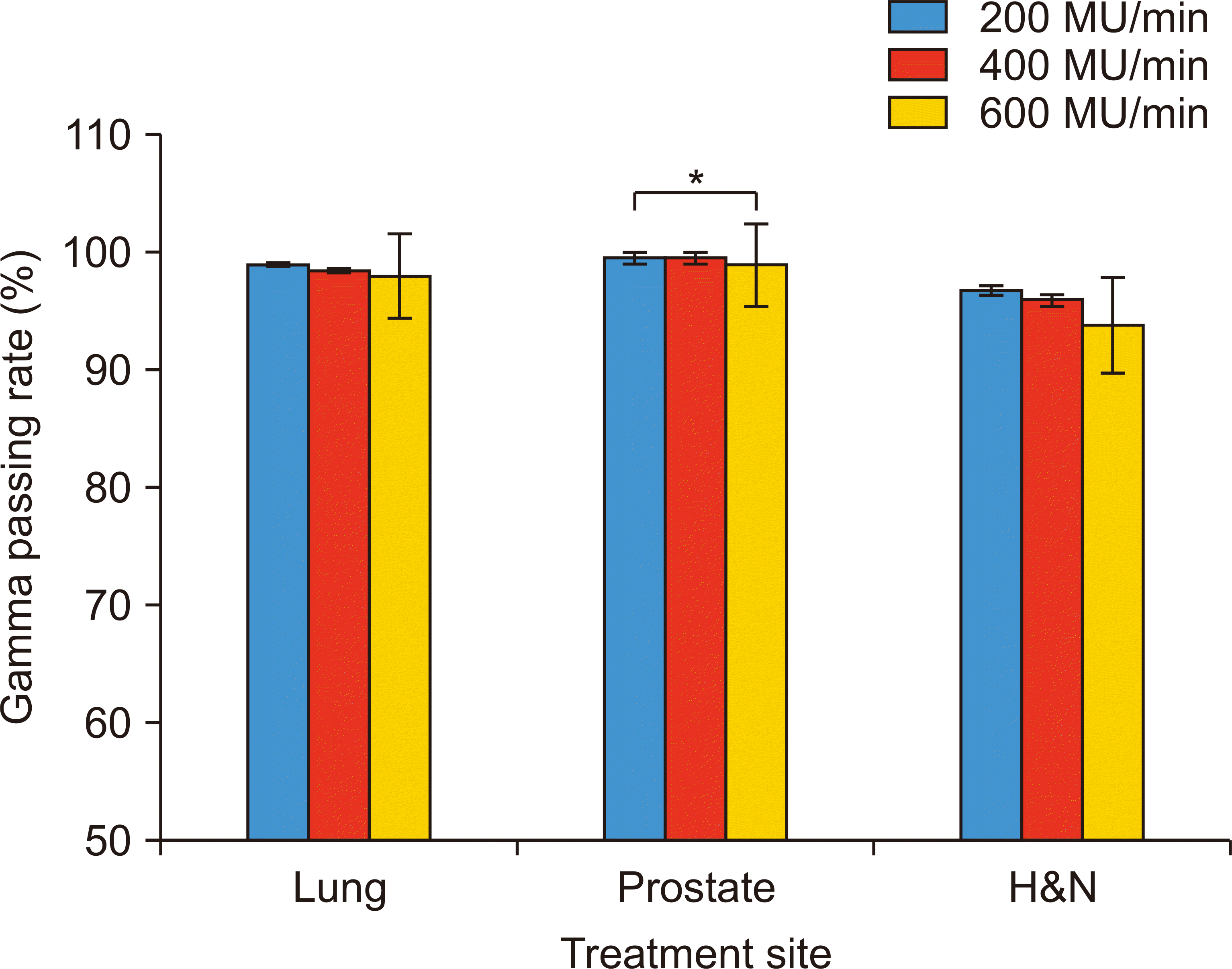
Fig. 3
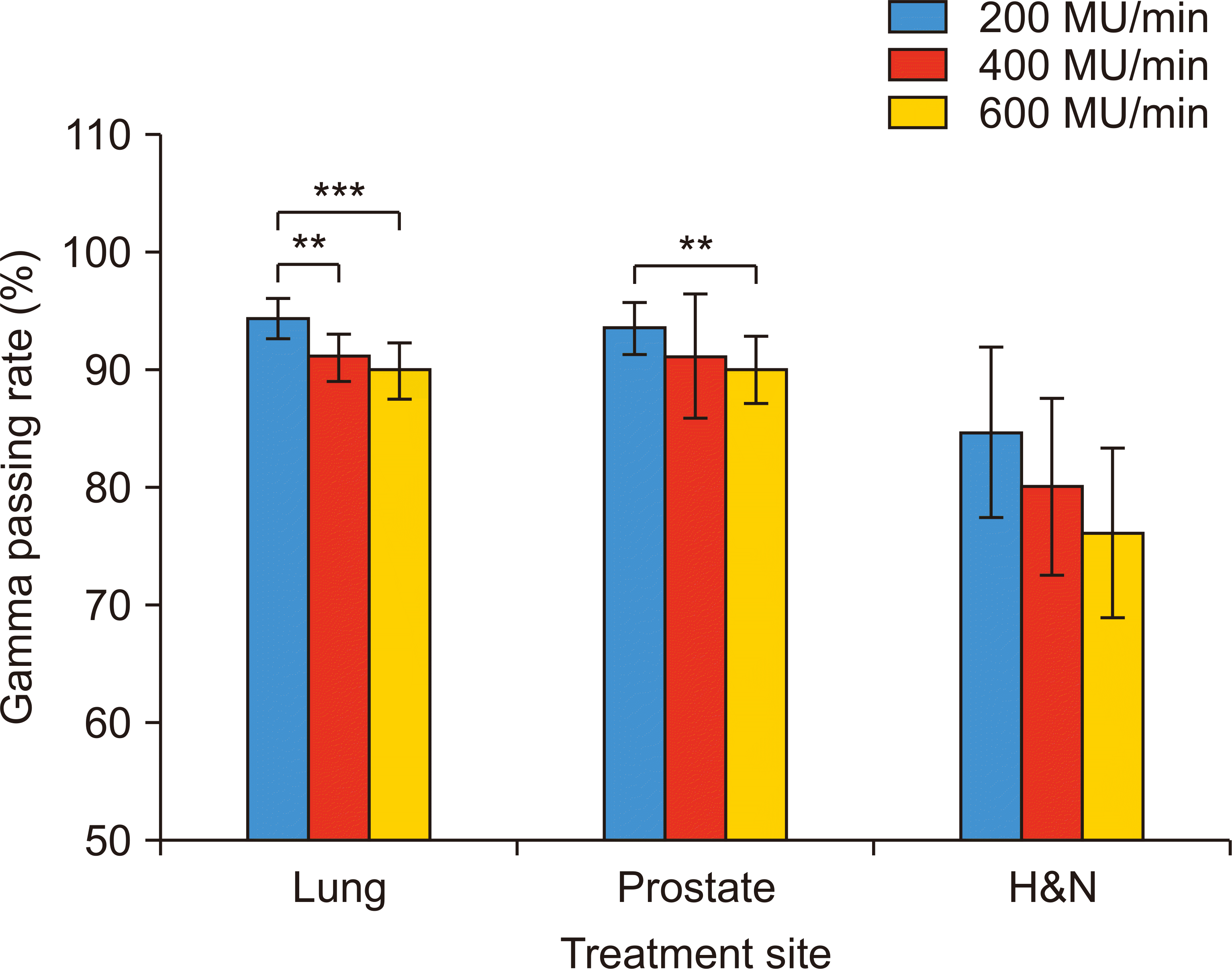
Gamma passing rate with the 2%/2-mm criterion as a function of dose rates for lung, prostate, and head and neck (H&N) disease sites (**P<0.01 and ***P<0.001).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download