Abstract
Objective
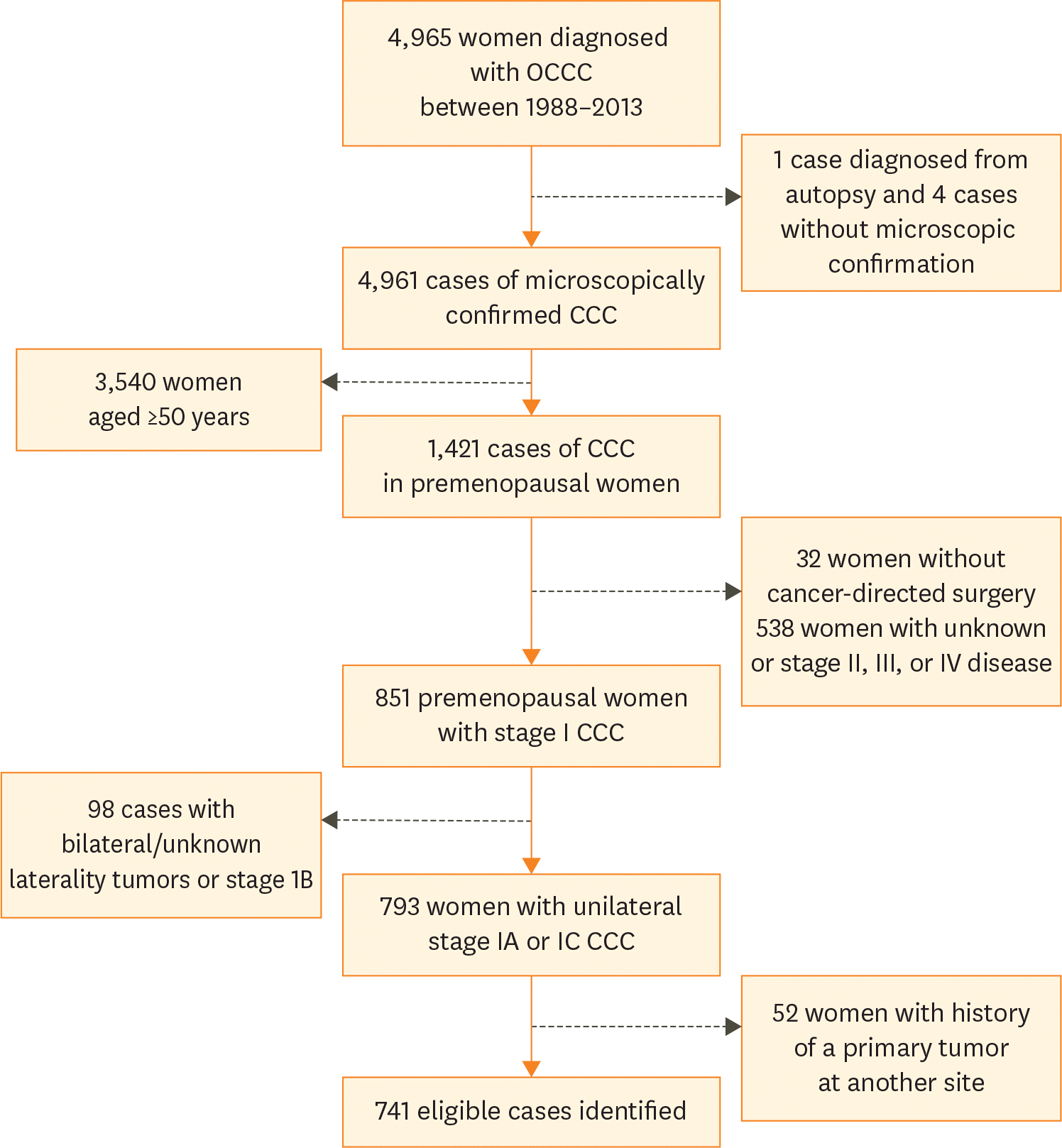
The aim of the present retrospective population-based study was to investigate the oncologic impact of uterine and ovarian preservation (OP) in premenopausal women with stage IA or IC ovarian clear cell carcinoma (OCCC).
Methods
The National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database was accessed and a cohort of surgically-staged premenopausal women (age <50 years) diagnosed with unilateral stage IA or IC OCCC was drawn. Based on site-specific surgery codes, women who did not undergo hysterectomy and/or bilateral salpingo-oophorectomy (BSO) were identified. Overall survival (OS) and cancer-specific survival (CSS) rates were calculated following generation of Kaplan-Meier curves; comparisons were made with the log-rank test. Multivariate Cox analysis was performed to control for possible confounders.
Results
A total of 741 premenopausal women who met the inclusion criteria were identified. Based on available information, rate of uterine preservation was 14.5% (96/663) while the rate of OP was 28.1% (71/253). Five-year CSS rates were 90.8% for women who did not undergo hysterectomy compared with 87.7% for those who did (p=0.290). Similarly, 5-year CSS rates in the OP and BSO groups were 92.6% and 85%, respectively (p=0.060). After controlling for disease sub-stage (IA vs. IC), uterine or OP was not associated with a worse overall or cancer-specific mortality.
References
1. Davidson B, Tropé CG. Ovarian cancer: diagnostic, biological and prognostic aspects. Womens Health (Lond). 2014; 10:519–33.

2. del Carmen MG, Birrer M, Schorge JO. Clear cell carcinoma of the ovary: a review of the literature. Gynecol Oncol. 2012; 126:481–90.

3. Mabuchi S, Sugiyama T, Kimura T. Clear cell carcinoma of the ovary: molecular insights and future therapeutic perspectives. J Gynecol Oncol. 2016; 27:e31.

4. Ditto A, Martinelli F, Lorusso D, Haeusler E, Carcangiu M, Raspagliesi F. Fertility sparing surgery in early stage epithelial ovarian cancer. J Gynecol Oncol. 2014; 25:320–7.

5. Tomao F, Peccatori F, Del Pup L, Franchi D, Zanagnolo V, Panici PB, et al. Special issues in fertility preservation for gynecologic malignancies. Crit Rev Oncol Hematol. 2016; 97:206–19.

6. Bentivegna E, Gouy S, Maulard A, Pautier P, Leary A, Colombo N, et al. Fertility-sparing surgery in epithelial ovarian cancer: a systematic review of oncological issues. Ann Oncol. 2016; 27:1994–2004.

7. Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013; 24(Suppl 6):vi24–32.

8. Lee YY, Kim TJ, Kim MJ, Kim HJ, Song T, Kim MK, et al. Prognosis of ovarian clear cell carcinoma compared to other histological subtypes: a meta-analysis. Gynecol Oncol. 2011; 122:541–7.

9. Satoh T, Hatae M, Watanabe Y, Yaegashi N, Ishiko O, Kodama S, et al. Outcomes of fertility-sparing surgery for stage I epithelial ovarian cancer: a proposal for patient selection. J Clin Oncol. 2010; 28:1727–32.

10. Park JY, Suh DS, Kim JH, Kim YM, Kim YT, Nam JH. Outcomes of fertility-sparing surgery among young women with FIGO stage I clear cell carcinoma of the ovary. Int J Gynaecol Obstet. 2016; 134:49–52.

11. Kajiyama H, Mizuno M, Shibata K, Yamamoto E, Kawai M, Nagasaka T, et al. Recurrence-predicting prognostic factors for patients with early-stage epithelial ovarian cancer undergoing fertility-sparing surgery: a multi-institutional study. Eur J Obstet Gynecol Reprod Biol. 2014; 175:97–102.

12. Kajiyama H, Shibata K, Mizuno M, Hosono S, Kawai M, Nagasaka T, et al. Fertility-sparing surgery in patients with clear-cell carcinoma of the ovary: is it possible? Hum Reprod. 2011; 26:3297–302.

13. Schilder JM, Thompson AM, DePriest PD, Ueland FR, Cibull ML, Kryscio RJ, et al. Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility-sparing therapy. Gynecol Oncol. 2002; 87:1–7.

14. National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Program (US). Incidence – SEER 18 regs research data + Hurricane Katrina impacted Louisiana cases, Nov 2015 Sub (1973–2013 varying) [Internet]. Bethesda, MD: National Cancer Institute;2015. [cited 2016 Sep 1]. Available from:. http://www.seer.cancer.gov.
15. Forman D, Bray F. Histological groups. Forman D, Bray F, Brewster DH, Mbalawa CG, Kohler B, Piñeros M, editors. editors.Cancer incidence in five continents Vol. X: IARC scientific publication no. 164. Lyon: International Agency for Research on Cancer;2014. p. 79–88.
16. Colombo N, Pecorelli S. What have we learned from ICON1 and ACTION? Int J Gynecol Cancer. 2003; 13(Suppl 2):140–3.

17. National Cancer Institute, Division of Cancer Control & Population Sciences (US). Joinpoint trend analysis software [Internet]. Bethesda, MD: National Cancer Institute;2017. [cited 2017 Apr 17]. Available from:. http://surveillance.cancer.gov/joinpoint.
18. Satoh T, Yoshikawa H. Fertility-sparing surgery for early stage epithelial ovarian cancer. Jpn J Clin Oncol. 2016; 46:703–10.

19. Okamoto A, Glasspool RM, Mabuchi S, Matsumura N, Nomura H, Itamochi H, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for clear cell carcinoma of the ovary. Int J Gynecol Cancer. 2014; 24:S20–5.

20. National Comprehensive Cancer Network (US). NCCN Clinical Practice Guidelines in Oncology. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer version 1.2016 [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;2016. [cited 2017 Feb 1]. Available from:. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
21. Satoh T, Tsuda H, Kanato K, Nakamura K, Shibata T, Takano M, et al. A non-randomized confirmatory study regarding selection of fertility-sparing surgery for patients with epithelial ovarian cancer: Japan Clinical Oncology Group Study (JCOG1203). Jpn J Clin Oncol. 2015; 45:595–9.

Fig. 2.
Rate of hysterectomy among premenopausal women with unilateral CCC confined to the ovary per year of diagnosis (final selected model: 0 joinpoints). APC, annual percent charge; CCC, clear cell carcinoma. * The APC is significantly different from zero at alpha=0.05.
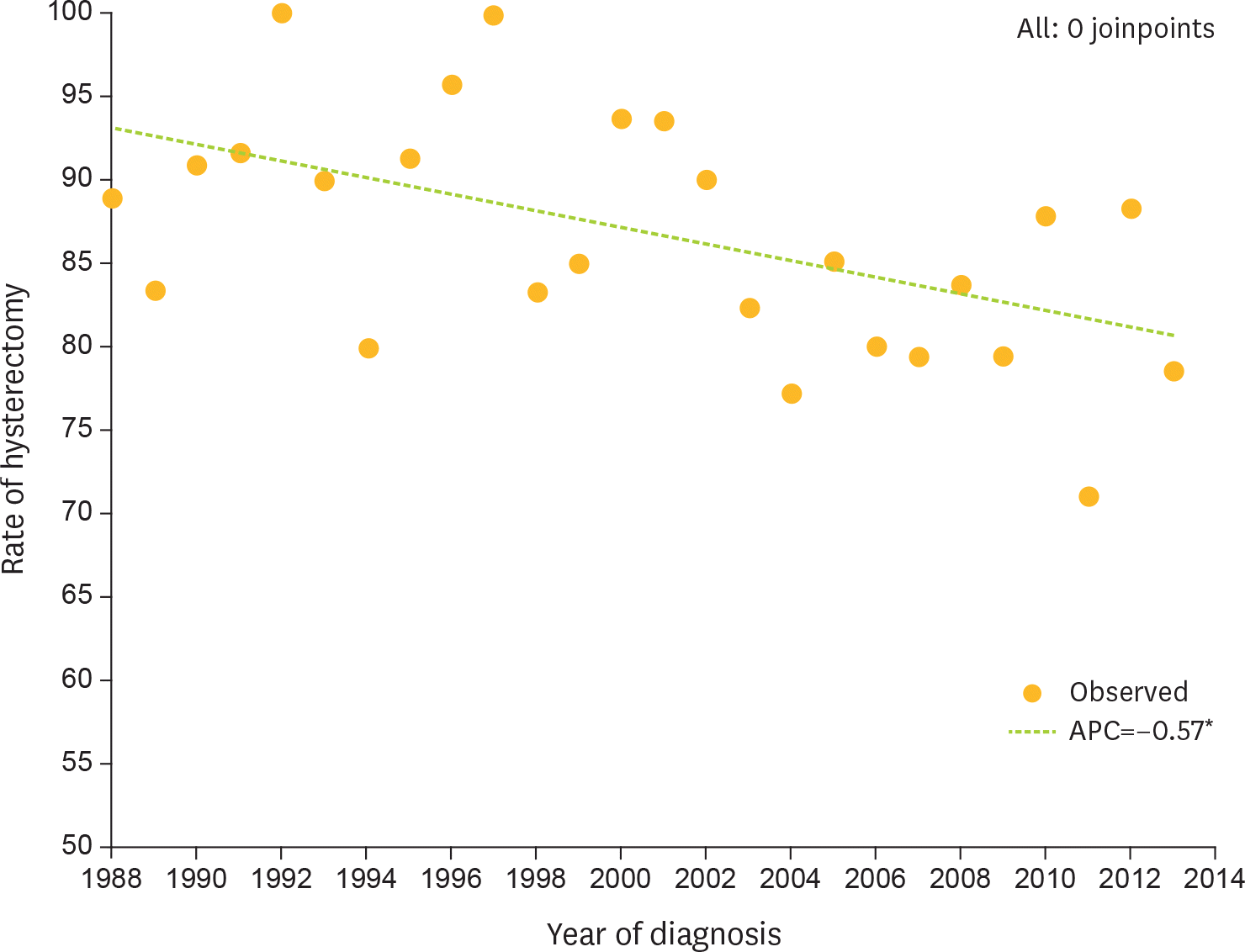
Fig. 3.
CSS of premenopausal women with unilateral CCC confined to the ovary stratified by performance of hysterectomy (n=564 and n=94 in the hysterectomy and uterine preservation groups, respectively; p=0.290 from the log-rank test). CCC, clear cell carcinoma; CSS, cancer-specific survival.
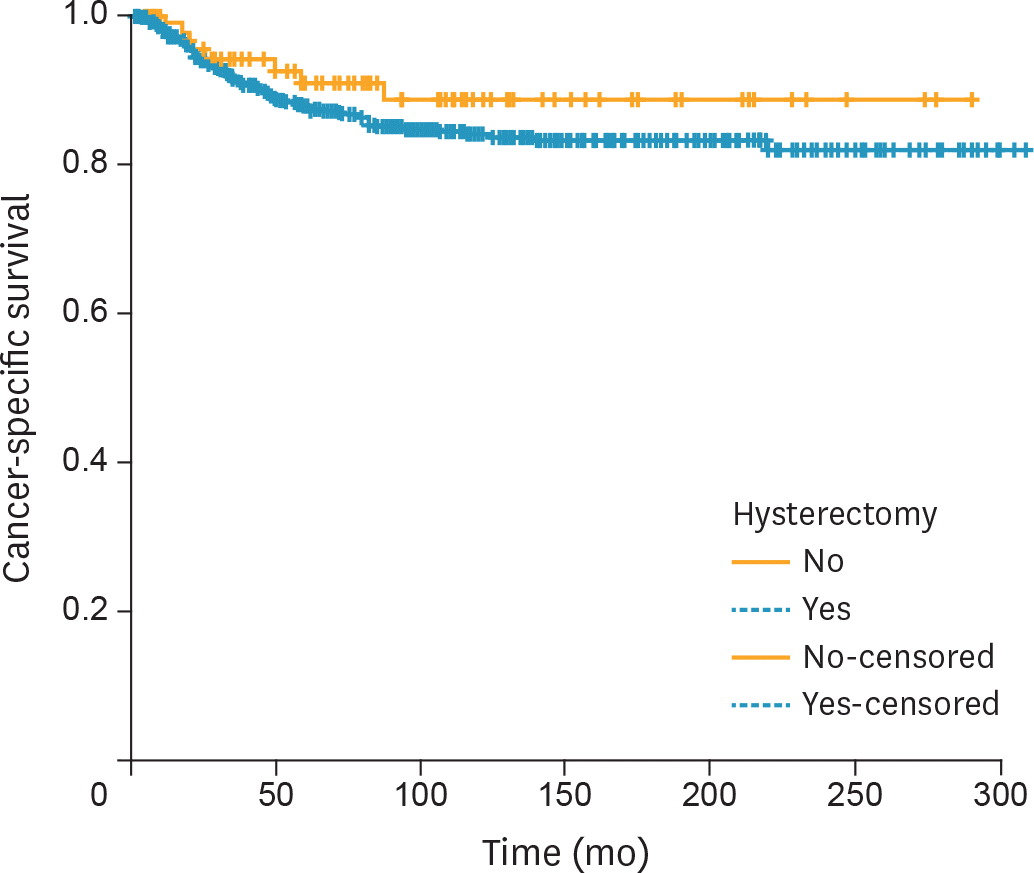
Fig. 4.
Rate of BSO among premenopausal women with unilateral CCC confined to the ovary per year of diagnosis (final selected model: 0 joinpoints). APC, annual percent charge; BSO, bilateral salpingo-oophorectomy; CCC, clear cell carcinoma. * The APC is significantly different from zero at alpha=0.05.
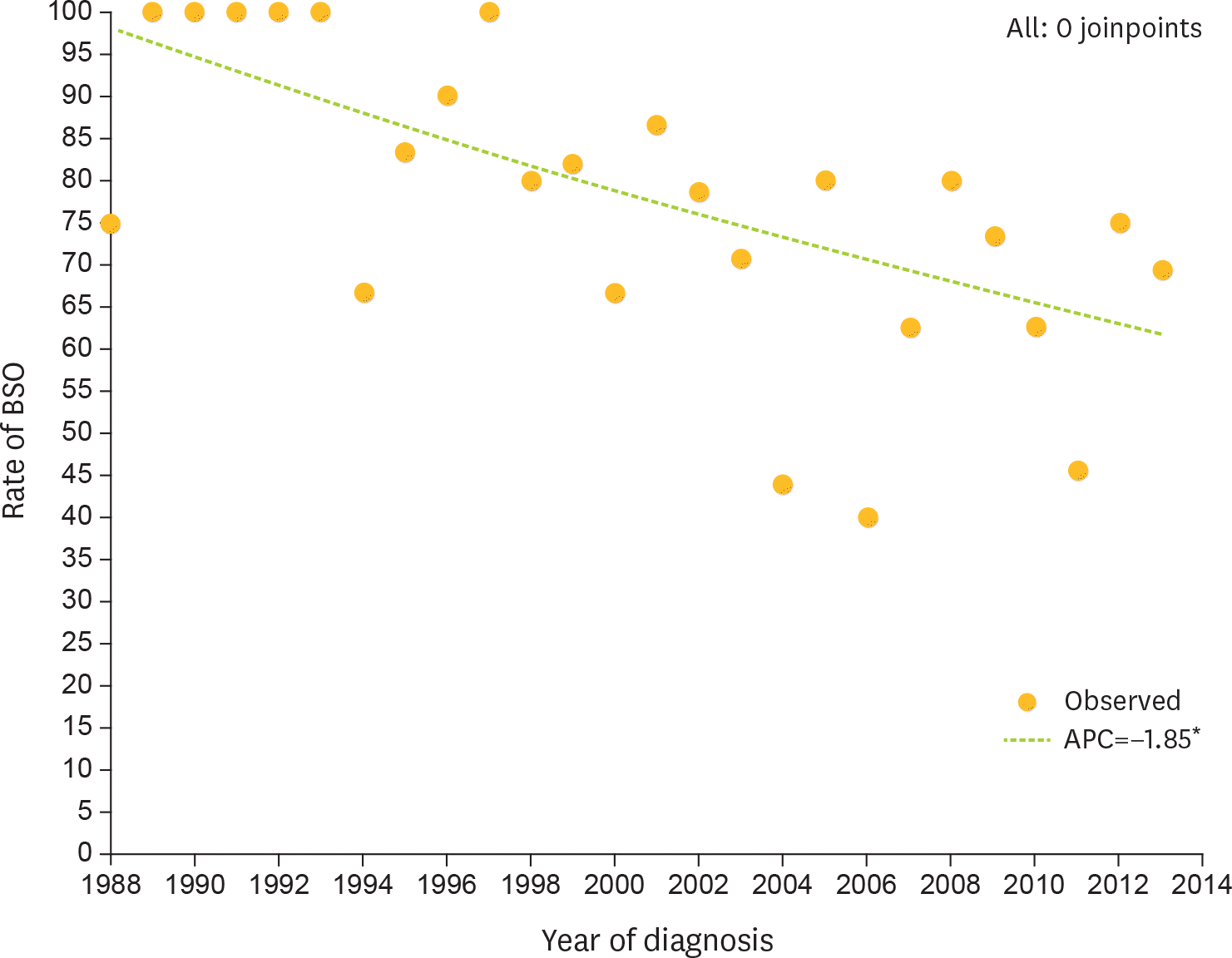
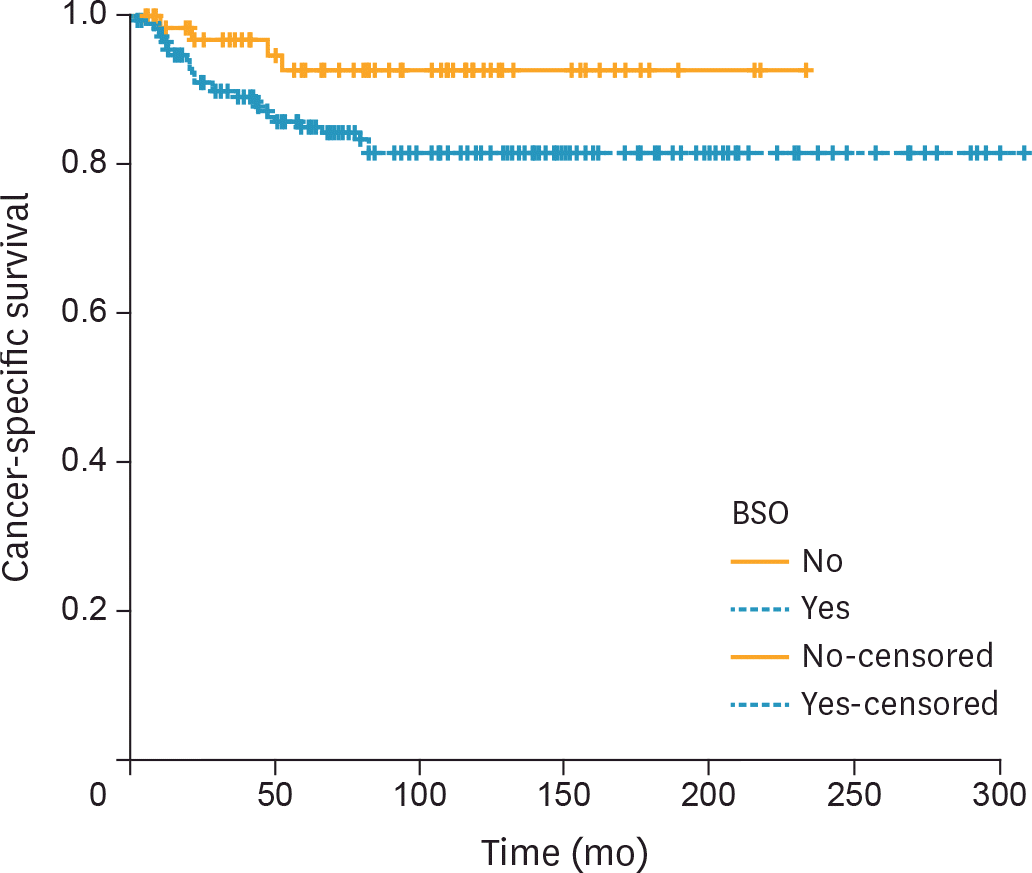
Fig. 5.
CSS of premenopausal women with unilateral CCC confined to the ovary stratified by performance of BSO (n=69 and n=181 in the USO and BSO groups, respectively; p=0.060 from the log-rank test). BSO, bilateral salpingo-oophorectomy; CCC, clear cell carcinoma; CSS, cancer-specific survival; USO, unilateral salpingo-oophorectomy.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download