1. Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965; 14:619–33.

2. Dinneen S, Alzaid A, Turk D, Rizza R. Failure of glucagon suppression contributes to postprandial hyperglycaemia in IDDM. Diabetologia. 1995; 38:337–43.

3. Muller WA, Faloona GR, Aguilar-Parada E, Unger RH. Abnormal alpha-cell function in diabetes: response to carbohydrate and protein ingestion. N Engl J Med. 1970; 283:109–15.
4. Greenbaum CJ, Prigeon RL, D’Alessio DA. Impaired beta-cell function, incretin effect, and glucagon suppression in patients with type 1 diabetes who have normal fasting glucose. Diabetes. 2002; 51:951–7.
5. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993; 329:977–86.

6. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003; 290:2159–67.
7. Lachin JM, Orchard TJ, Nathan DM; DCCT/EDIC Research Group. Update on cardiovascular outcomes at 30 years of the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014; 37:39–43.

8. Luddeke HJ, Sreenan S, Aczel S, Maxeiner S, Yenigun M, Kozlovski P, et al. PREDICTIVE: a global, prospective observational study to evaluate insulin detemir treatment in types 1 and 2 diabetes: baseline characteristics and predictors of hypoglycaemia from the European cohort. Diabetes Obes Metab. 2007; 9:428–34.
9. Donnelly LA, Morris AD, Frier BM, Ellis JD, Donnan PT, Durrant R, et al. Frequency and predictors of hypoglycaemia in type 1 and insulin-treated type 2 diabetes: a population-based study. Diabet Med. 2005; 22:749–55.

10. Chillaron JJ, Flores Le-Roux JA, Benaiges D, Pedro-Botet J. Type 1 diabetes, metabolic syndrome and cardiovascular risk. Metabolism. 2014; 63:181–7.

11. Rodrigues TC, Veyna AM, Haarhues MD, Kinney GL, Rewers M, Snell-Bergeon JK. Obesity and coronary artery calcium in diabetes: the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study. Diabetes Technol Ther. 2011; 13:991–6.

12. Pambianco G, Costacou T, Orchard TJ. The prediction of major outcomes of type 1 diabetes: a 12-year prospective evaluation of three separate definitions of the metabolic syndrome and their components and estimated glucose disposal rate: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes Care. 2007; 30:1248–54.

13. Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care. 2006; 29:2528–38.

14. Purnell JQ, Dev RK, Steffes MW, Cleary PA, Palmer JP, Hirsch IB, et al. Relationship of family history of type 2 diabetes, hypoglycemia, and autoantibodies to weight gain and lipids with intensive and conventional therapy in the Diabetes Control and Complications Trial. Diabetes. 2003; 52:2623–9.

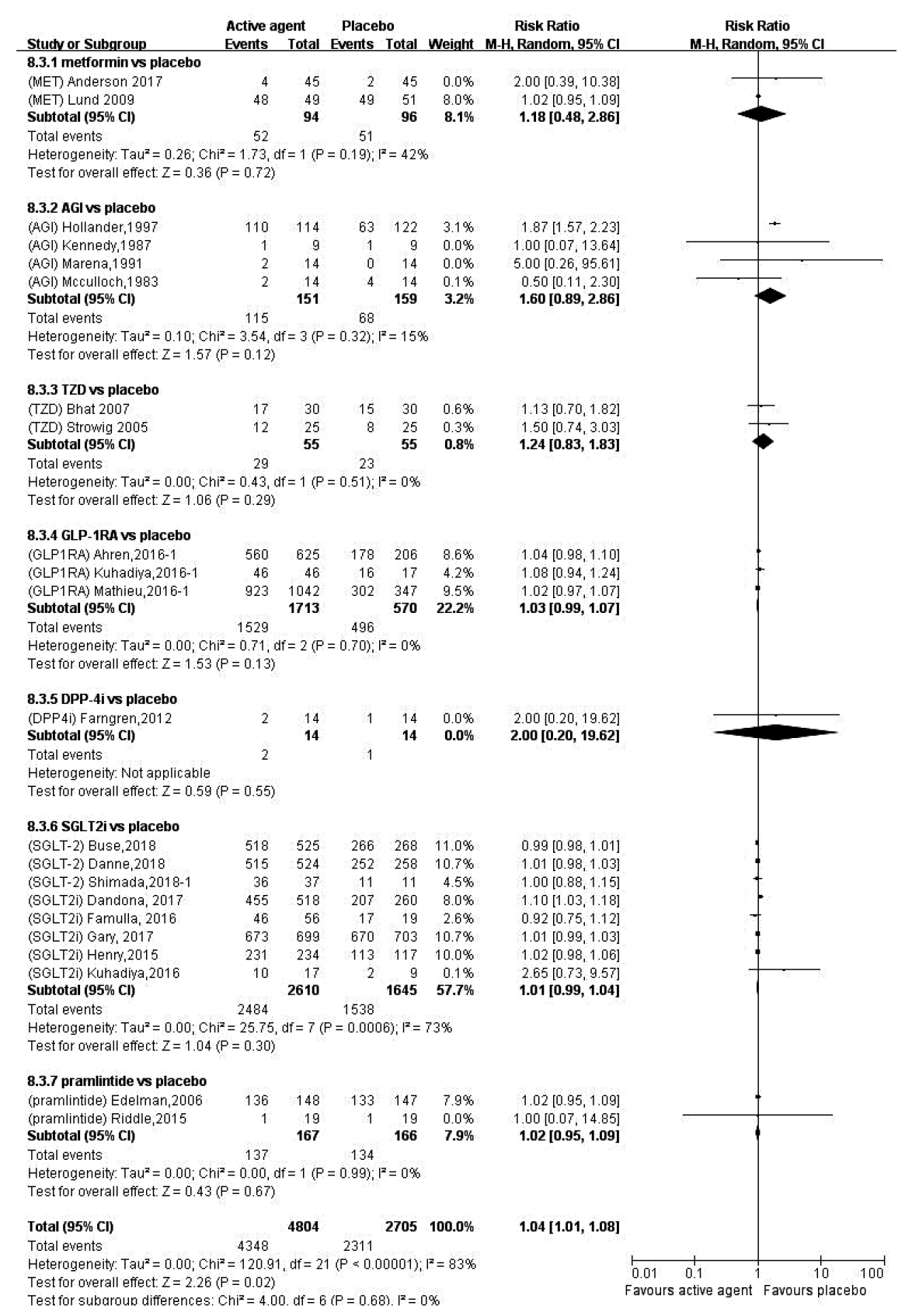
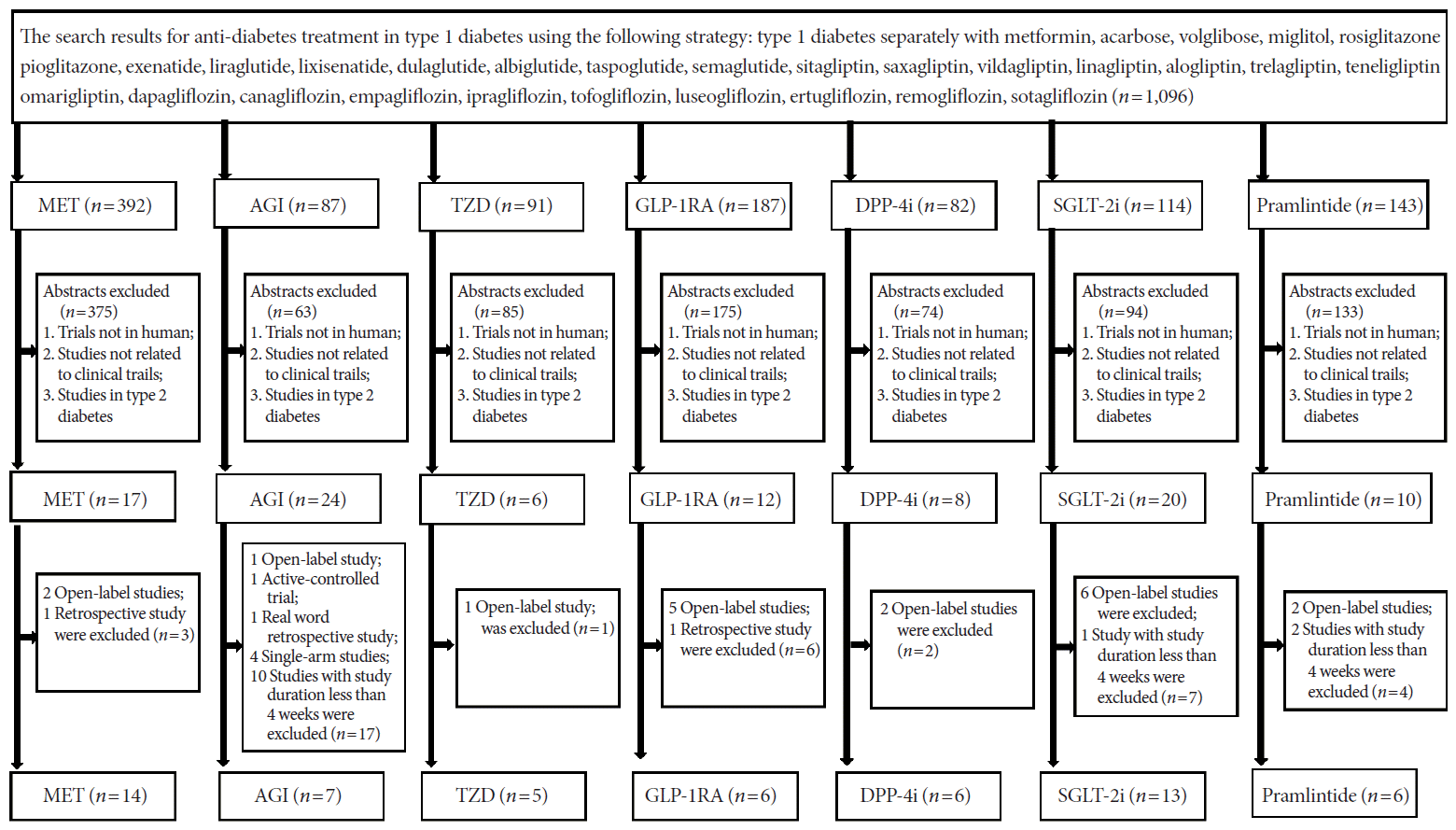
15. Frandsen CS, Dejgaard TF, Madsbad S. Non-insulin drugs to treat hyperglycaemia in type 1 diabetes mellitus. Lancet Diabetes Endocrinol. 2016; 4:766–80.

16. Bode BW, Garg SK. The emerging role of adjunctive noninsulin antihyperglycemic therapy in the management of type 1 diabetes. Endocr Pract. 2016; 22:220–30.

17. Pettus J, Hirsch I, Edelman S. GLP-1 agonists in type 1 diabetes. Clin Immunol. 2013; 149:317–23.

18. Harris KB, Boland CL. Adjunctive role of glucagon-like peptide-1 receptor agonists in the management of type 1 diabetes mellitus. Pharmacotherapy. 2016; 36:1011–20.

19. Munir KM, Davis SN. The treatment of type 1 diabetes mellitus with agents approved for type 2 diabetes mellitus. Expert Opin Pharmacother. 2015; 16:2331–41.

20. Wang W, Liu H, Xiao S, Liu S, Li X, Yu P. Effects of insulin plus glucagon-like peptide-1 receptor agonists (GLP-1RAs) in treating type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther. 2017; 8:727–38.

21. Guo H, Fang C, Huang Y, Pei Y, Chen L, Hu J. The efficacy and safety of DPP4 inhibitors in patients with type 1 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2016; 121:184–91.

22. Livingstone R, Boyle JG, Petrie JR; REMOVAL Study Team. A new perspective on metformin therapy in type 1 diabetes. Diabetologia. 2017; 60:1594–600.

23. Garg SK, Michels AW, Shah VN. Use of non-insulin therapies for type 1 diabetes. Diabetes Technol Ther. 2013; 15:901–8.

24. Chen J, Fan F, Wang JY, Long Y, Gao CL, Stanton RC, et al. The efficacy and safety of SGLT2 inhibitors for adjunctive treatment of type 1 diabetes: a systematic review and meta-analysis. Sci Rep. 2017; 7:44128.

25. Heller SR, Amiel SA, Mansell P. Effect of the fast-acting insulin analog lispro on the risk of nocturnal hypoglycemia during intensified insulin therapy. U.K. Lispro Study Group. Diabetes Care. 1999; 22:1607–11.

26. Adverse events and their association with treatment regimens in the diabetes control and complications trial. Diabetes Care. 1995; 18:1415–27.
27. Polsky S, Ellis SL. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2015; 22:277–82.

28. Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ. Changing prevalence of overweight children and adolescents at onset of insulin-treated diabetes. Diabetes Care. 2003; 26:2871–5.

29. Liu LL, Lawrence JM, Davis C, Liese AD, Pettitt DJ, Pihoker C, et al. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes. 2010; 11:4–11.

30. DuBose SN, Hermann JM, Tamborlane WV, Beck RW, Dost A, DiMeglio LA, et al. Obesity in youth with type 1 diabetes in Germany, Austria, and the United States. J Pediatr. 2015; 167:627–32.
31. Klupa T. Metformin in type 1 diabetes mellitus?: revisiting treatment dogmas in diabetes. Pol Arch Med Wewn. 2016; 126:461–2.

32. Priya G, Kalra S. A review of insulin resistance in type 1 diabetes: is there a place for adjunctive metformin? Diabetes Ther. 2018; 9:349–61.

33. Frohlich-Reiterer EE, Rosenbauer J, Bechtold-Dalla Pozza S, Hofer SE, Schober E, Holl RW, et al. Predictors of increasing BMI during the course of diabetes in children and adolescents with type 1 diabetes: data from the German/Austrian DPV multicentre survey. Arch Dis Child. 2014; 99:738–43.

34. Mortensen HB, Robertson KJ, Aanstoot HJ, Danne T, Holl RW, Hougaard P, et al. Insulin management and metabolic control of type 1 diabetes mellitus in childhood and adolescence in 18 countries. Hvidore Study Group on Childhood Diabetes. Diabet Med. 1998; 15:752–9.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download