INTRODUCTION
METHODS
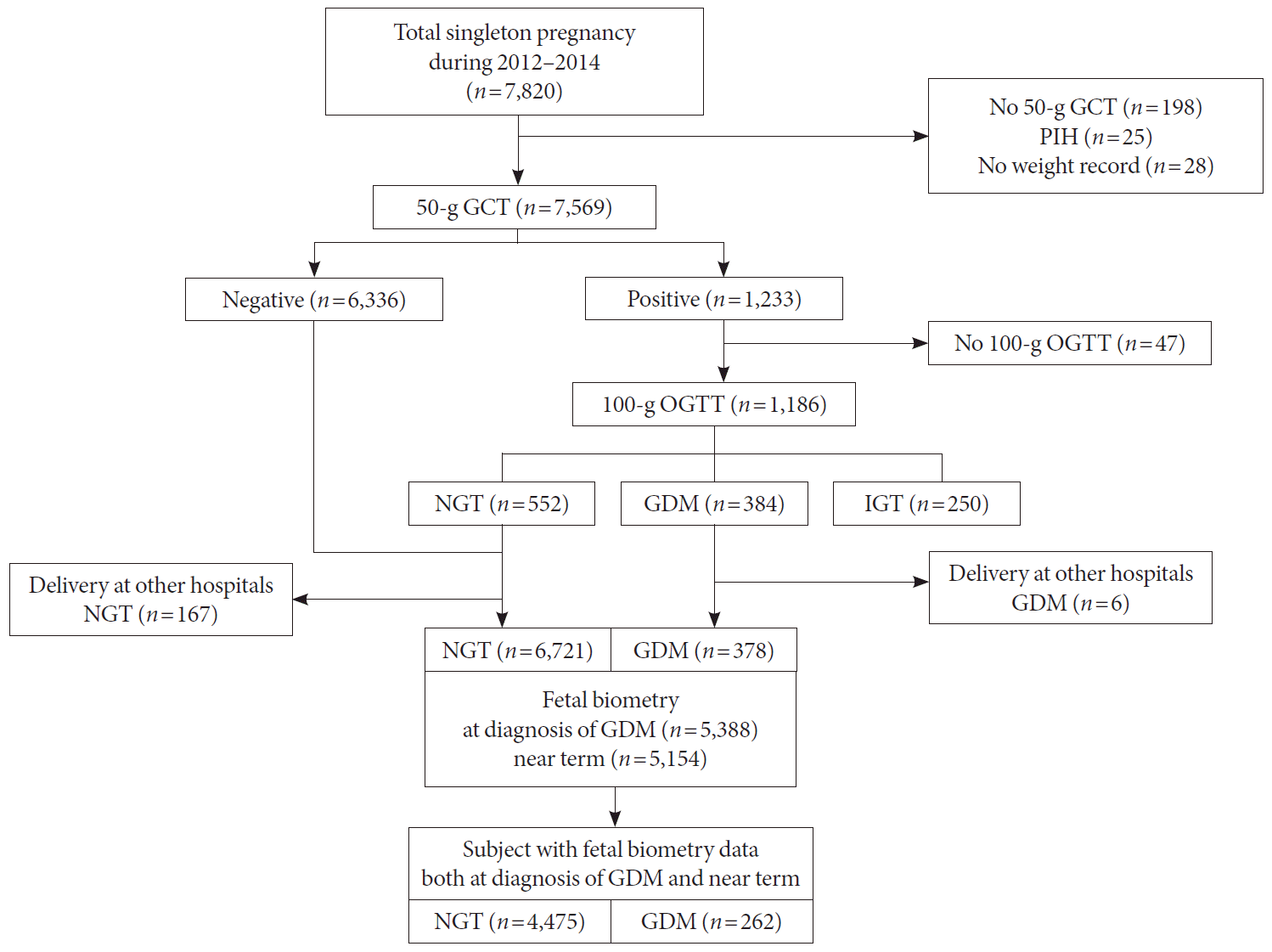
Subjects and data collection
Diagnosis of GDM
Treatment of GDM
Fetal biometry
Biochemical analysis
Subgroup analysis of GDM subjects according to maternal age and prepregnancy BMI
Subgroup analysis of the NGT and GDM subjects according to presence or absence of FAO near term
Statistical analyses
RESULTS
Clinical and biochemical characteristics and pregnancy outcomes of the NGT and GDM subjects
Table 1.
| Characteristic | NGT (n=6,721) | Total GDM (n=378) |
GDM |
|||
|---|---|---|---|---|---|---|
| Group 1 (n=144) | Group 2 (n=22) | Group 3 (n=162) | Group 4 (n=50) | |||
| Age, yr | 33.1±3.8 | 35.3±4.0a | 31.7±1.7a | 31.4±2.2 | 38.0±2.6a.b,c | 38.6±2.6a,b,c |
| Prepregnancy BMI, kg/m2 | 20.6±2.6 | 22.3±3.5a | 20.6±2.0 | 28.0±2.8a,b | 21.3±2.0a,c | 28.0±2.4a,b,d |
| Weight gain, kg | ||||||
| Pre-pregnancy−at diagnosis | 7.6±3.2 | 7.8±3.6 | 8.3±3.7 | 6.7±4.3a | 8.0±3.4 | 6.9±3.4a |
| Pre-pregnancy−near term | 13.0±4.1 | 11.5±4.6a | 12.2±4.5 | 9.3±6.4a,b | 11.7±4.1a | 9.5±4.8a,b |
| Insulin treatment, % | - | 10.4 | 8.2 | 17.4b | 9.8 | 15.7b |
| Mean plasma glucose, mg/dL | ||||||
| Fasting | - | 81.9±9.0 | 80.8±7.8 | 89.5±16.3b | 80.9±7.5c | 85.4±9.9b,d |
| Postprandial 1 hour | - | 133.0±19.1 | 133.2±18.0 | 140.5±25.3 | 129.7±17.1 | 140.7±22.8b,d |
| Mean glycated albumin, % | - | 11.8±1.4 | 11.7±1.3 | 11.6±1.3 | 12.0±1.4 | 11.7±1.7 |
| HbA1c, % | ||||||
| At diagnosis | 5.0±0.3 | 5.3±0.5a | 5.2±0.3a | 5.6±0.6a,b | 5.2±0.3a,c | 5.6±1.0a,b,d |
| Near term | - | 5.6±0.6 | 5.5±0.4 | 5.9±0.5b | 5.5±0.4c | 5.8±0.7b,d |
| Pregnancy outcomes | ||||||
| Primipara, % | 70.2 | 66.7 | 77.1 | 81.8 | 59.9a | 52.0a |
| Primary cesarean delivery, % | 22.5 | 30.2a | 22.9 | 36.4 | 35.8a | 30.0 |
| Infant birth weight, g | 3,197±421 | 3,226±510 | 3,157±487 | 3,303±677b | 3,262±452a,b | 3,265±654 |
| GA at delivery, wk | 39.0±1.5 | 38.4±1.8a | 38.5±1.8a | 38.2±2.2a | 38.5±1.5a | 37.8±2.4a |
| LGA, % | 5.1 | 12.7a | 7.6 | 18.2a | 14.2a | 20.0a,b |
| Macrosomia, %e | 1.9 | 4.5a | 3.5 | 4.6 | 4.3a | 8.0a |
Values are presented as mean±standard deviation. Group 1 (age <35 years and BMI <25 kg/m2); Group 2 (age <35 years and BMI ≥25 kg/m2); Group 3 (age ≥35 years and BMI <25 kg/m2); Group 4 (age ≥35 years and BMI ≥25 kg/m2).
NGT, normal glucose tolerance; GDM, gestational diabetes mellitus; BMI, body mass index; HbA1c, glycosylated hemoglobin; GA, gestational age; LGA, large for gestational age.
Fetal biometry of the NGT and GDM subjects
Table 2.
| Variable | NGT (n=4,822) | Total GDM (n=332) |
GDM |
|||
|---|---|---|---|---|---|---|
| Group 1 (n=125) | Group 2 (n=21) | Group 3 (n=144) | Group 4 (n=42) | |||
| Fetal biometry | ||||||
| GA-LMP, wkd | 35.9±1.3 | 35.6±1.6b | 35.4±1.6b | 35.8±1.3 | 35.8±1.5 | 35.4±1.8a |
| GA-BPD, wk | 37.2±2.1 | 36.9±2.3a | 36.7±2.2a | 37.4±2.4 | 37.1±2.3 | 36.6±2.6a |
| GA-AC, wk | 38.0±2.2 | 38.3±2.7a | 37.6±2.8a | 39.1±2.3a | 38.7±2.5b | 38.4±2.6 |
| GA-FL, wk | 36.9±1.8 | 36.7±1.9 | 36.4±2.0b | 37.4±1.4 | 36.9±1.8 | 36.6±2.1 |
| EFW, g | 2,653±305 | 2,660±370 | 2,576±377a | 2,780±338 | 2,717±354a | 2,651±380 |
| FAOR | ||||||
| GA-AC/GA-LMPd | 1.06±0.05 | 1.08±0.06c | 1.06±0.06 | 1.09 ±0.05a | 1.08±0.06c | 1.09±0.05b |
| GA-AC/GA-BPD | 1.02±0.06 | 1.04±0.06c | 1.03±0.07 | 1.05±0.06 | 1.05±0.06c | 1.05±0.07b |
| GA-AC/GA-FL | 1.03±0.05 | 1.04±0.06c | 1.04±0.06 | 1.05±0.06 | 1.05±0.06c | 1.05±0.05a |
Values are presented as mean±standard deviation. Group 1 (age <35 years and BMI <25 kg/m2); Group 2 (age <35 years and BMI ≥25 kg/m2); Group 3 (age ≥35 years and BMI <25 kg/m2); Group 4 (age ≥35 years and BMI ≥25 kg/m2).
FAOR, fetal abdominal overgrowth ratio; NGT, normal glucose tolerance; GDM, gestational diabetes mellitus; BMI, body mass index; GA-LMP, gestational age by last menstruation period; GA-BPD, estimated gestational age by biparietal diameter; GA-AC, estimated gestational age by abdominal circumference; GA-FL, estimated gestational age by femur length; EFW, estimated fetal weight.
Odds ratio for exhibiting FAO near term in subjects with GDM and subgroups of GDM
Table 3.
| Variable |
FAO, OR (95% CI)a |
||
|---|---|---|---|
| GA-AC/GA-LMPb ≥90th | GA-AC/GA-BPD ≥90th | GA-AC/GA-FL ≥90th | |
| NGT | 1 (reference) | 1 (reference) | 1 (reference) |
| Total GDM | 2.12 (1.57–2.85)c | 2.08 (1.54–2.81)c | 1.69 (1.23–2.31)c |
| Group 1 GDM | 1.14 (0.64–2.04) | 1.87 (1.15–3.05)c | 1.46 (0.87–2.46) |
| Group 2 GDM | 3.92 (1.24–10.75)c | 2.31 (0.56–7.13) | 1.55 (0.29–5.34) |
| Group 3 GDM | 2.47 (1.62–3.76)c | 1.87 (1.18–2.95)c | 1.86 (1.19–2.91)c |
| Group 4 GDM | 3.48 (1.74–6.96)c | 3.49 (1.74–6.98)c | 1.86 (0.82–4.21) |
OR, odds ratio; FAO, fetal abdominal obesity; FAOR, fetal abdominal overgrowth ratio; GDM, gestational diabetes mellitus; NGT, normal glucose tolerance; CI, confidence interval; GA-AC, estimated gestational age by abdominal circumference; GA-LMP, gestational age by last menstruation period; GA-BPD, estimated gestational age by biparietal diameter; GA-FL, estimated gestational age by femur length.
Odds ratios for exhibiting FAO near term, being LGA at birth, and macrosomia
Table 4.
| Variable |
Neonatal outcomes, OR (95% CI) |
||
|---|---|---|---|
| FAO near term (+) | LGA | Macrosomia | |
| NGT | |||
| FAOc at diagnosis (–) | 1 (reference) | 1 (reference) | 1 (reference) |
| FAO at diagnosis (+) | 4.65 (3.64–5.94)a | 4.56 (3.35–6.21)a | 4.45 (2.75–7.20)a |
| GDM | |||
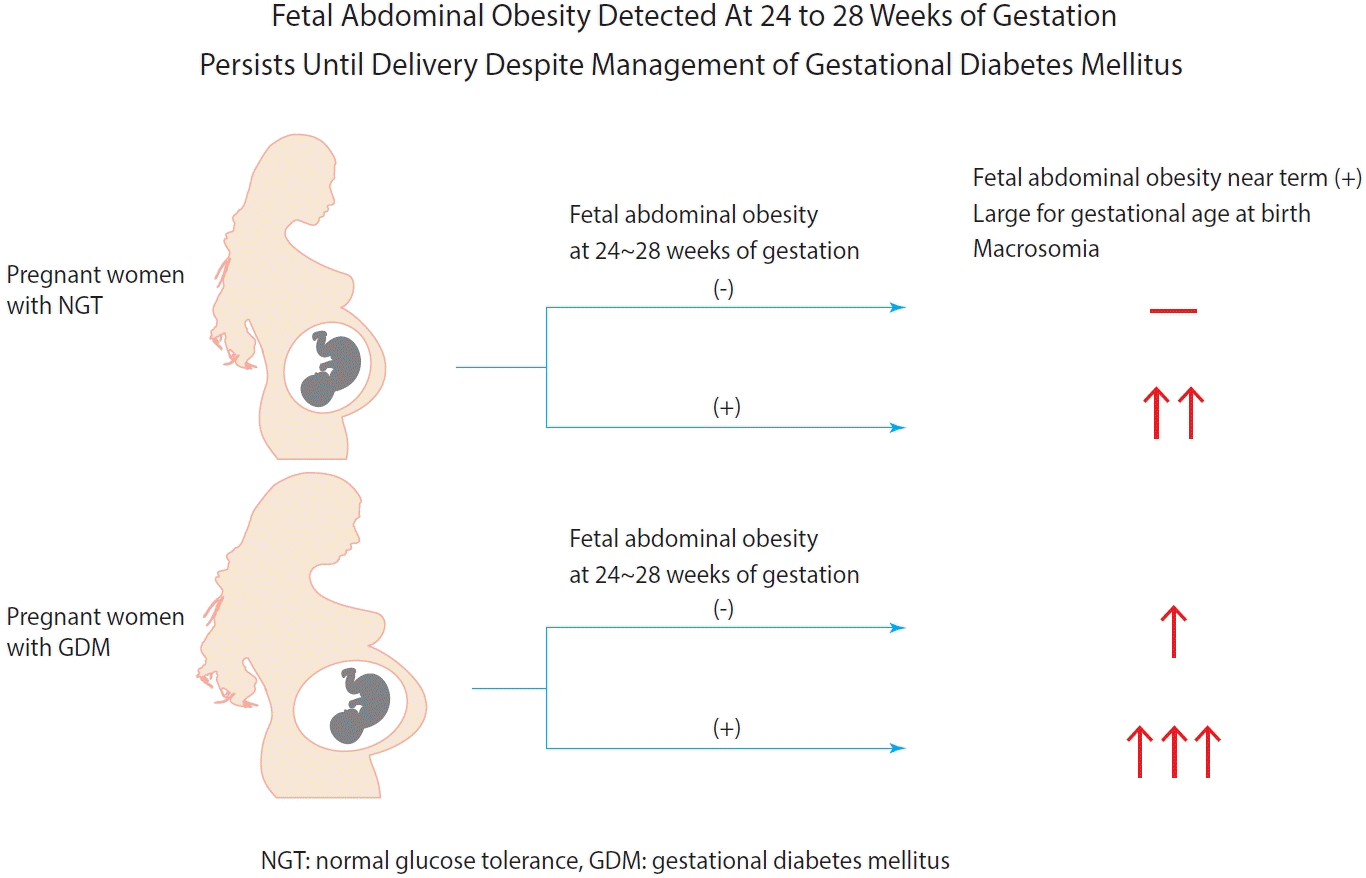
| FAO at diagnosis (–) | 1.73 (1.13–2.65)b | 2.72 (1.68–4.38)a | 3.82 (1.97–7.40)a |
| FAO at diagnosis (+) | 7.87 (4.38–14.15)a | 10.96 (5.58–20.53)a | 4.58 (1.38–15.14)b |
Clinical characteristics of GDM and NGT subjects based on presence or absence of FAO near term
Table 5.
|
NGT |
GDM |
|||
|---|---|---|---|---|
| FAOa (–) (n=4,059) | FAO (+) (n=416) | FAO (–) (n=217) | FAO (+) (n=45) | |
| Age, yr | 33.1±3.7 | 33.7±3.5b | 35.2±3.9 | 36.4±4.3 |
| Prepregnancy BMI, kg/m2 | 20.5±2.5 | 21.6±3.1b | 22.0±3.1 | 23.7±4.0b |
| Weight gain, kg | ||||
| Pre-pregnancy−at diagnosis | 7.5±3.1 | 8.3±4.2b | 7.5±3.4 | 8.6±4.2 |
| Pre-pregnancy−near term | 12.9±4.0 | 13.9±4.4b | 11.3±4.3 | 12.0±5.7 |
| Insulin treatment, % | - | - | 9.2 | 22.2b |
| FPG on 100-g OGTT, mg/dL | 79.9±6.2 | 82.0±6.6 | 89.0±15.8 | 93.7±13.6 |
| Mean plasma glucose, mg/dL | ||||
| Fasting | - | 81.0±7.0 | 86.8±14.0b | |
| Postprandial 1 hour | - | 131.8±18.9 | 137.4±25.4 | |
| Mean glycated albumin, % | - | - | 11.8±1.4 | 11.9±1.2 |
| HbA1c, % | ||||
| At diagnosis | 5.0±0.3 | 5.0±0.3 | 5.3±0.5 | 5.5±0.6b |
| Near term | - | - | 5.5±0.5 | 5.7±0.6 |
| Pregnancy outcomes | ||||
| Primipara, % | 66.8 | 60.8b | 66.4 | 60.0 |
| Primary cesarean delivery, % | 22.7 | 28.8b | 29.7 | 37.8 |
| Infant birth weight, g | 3,174±366 | 3,574±383b | 3,189±425 | 3,670±362b |
| GA at delivery, wk | 39.1±1.2 | 38.8±1.4b | 38.6±1.5 | 38.3±1.2 |
| LGA, % | 3.0 | 26.1b | 7.5 | 44.4b |
| Macrosomia, % | 1.0 | 10.1b | 3.2 | 15.6b |
Values are presented as mean±standard deviation.
NGT, normal glucose tolerance; GDM, gestational diabetes mellitus; FAO, fetal abdominal obesity; BMI, body mass index; FPG, fasting plasma glucose; OGTT, oral glucose tolerance test; HbA1c, glycosylated hemoglobin; GA, gestational age; LGA, large for gestational age.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download