INTRODUCTION
As of May 2021, the coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory coronavirus 2 (SARS-CoV-2), has accumulated approximately 170 million confirmed cases and more than 3.5 million deaths worldwide.
1 This has had a devastating effect on the economy and on society at large.
2 Therefore, several countries have invested significantly in the development and supply of vaccines. South Korea began implementing its nationwide COVID-19 vaccination program on February 26, 2021. Vaccines approved for use in South Korea include the AstraZeneca recombinant adenoviral ChAdOx1-S, the Pfizer/BioNTech BNT162B2, and the Janssen Ad26.COV2.S vaccines; however, the first two are the most being administered in the early stage. As part of this vaccination program, Korean healthcare workers (HCWs) received the first doses of either the ChAdOx1 nCoV-19 or the BNT162b2 vaccines in March 2021. The second stage of vaccination, targeting healthy individuals aged 18-64 is currently underway.
3
The most common systemic reactions to the ChAdOx1-S and the BNT162B2 vaccines are muscle aches (77.7%), fatigue (74.7%), headaches (67.4%), chills (63.5%), and fever (49.2%).
4 Since reports have emerged of thrombotic adverse events stemming from application of the ChAdOx1-S vaccine,
5 there are concerns among the Korean public regarding these new vaccination programs. People who suffer from these adverse events may decide to visit a hospital's emergency department (ED).
During the COVID-19 pandemic, when patients have reported having a fever or exhibiting a systemic response that resembles COVID-19 symptomatology, treatment is performed in an isolated space separate from other patients. Many ED staff members have reported struggling to secure enough quarantine space for the treatment of febrile patients. Importantly, most systemic symptoms of post-vaccination patients resemble COVID-19 symptomatology
467; therefore, if nationwide vaccination begins, additional overcrowding is expected in the EDs.
7 Despite these concerns, studies on the adverse post-vaccination events are being conducted, but studies on these events, specifically within the context of ED utilization, remain scarce.
This is an observational study seeking to analyze the utilization pattern of a post-vaccination population in a single ED where fast-track and “post-vaccination cohort zone (PVCZ)” are preemptively introduced.
METHODS
Study design and population
We conducted a retrospective observational study in a tertiary teaching hospital whose ED serves as a regional emergency center in a southern metropolitan area of South Korea. This ED had an average of 90,000 visits annually before the COVID-19 outbreak. The hospital currently operates as an isolation facility for severely ill COVID-19 patients, and also serves as a COVID-19 vaccination center.
We included all in-hospital HCWs who were administered the first COVID-19 vaccination dose between March 4 and April 2, 2021. Most vaccinations were administered from March 4–14, 2021, and from March 25–27, 2021, subsequently, additional vaccination dates were added (March 16 and April 1–2) for new employees. We implemented a fast-track system, designated a PVCZ in the ED during the in-hospital vaccination period, and retrospectively reviewed the electronic medical records of individuals who visited the ED within one-week post-vaccination.
The COVID-19 vaccination protocol and the fast-track system in ED
To properly classify ED patients during the COVID-19 pandemic, we changed the triage system according to patients' symptoms and chest X-ray (CXR) results. This classification system was used to categorize patients into five different zones: the resuscitation, negative–pressure isolation, febrile cohort, non-febrile cohort, and pediatric zones. PVCZ was operated in the ED to avoid overcrowding and unnecessary COVID-19 diagnostic work from March 4–17, 2021 and March 25–April 4, 2021, on days scheduled for HCWs' vaccination and post-vaccination period.
Considering that the most side effects of COVID-19 vaccines appear within 48 hours after administration, the fast-track system was implemented. After vaccination, people were observed for 15-20 minutes in a specific space to check for any hyper acute side effects such as anaphylaxis. If no such side effects appeared during this observation time, they were allowed to leave and were provided with a pamphlet detailing common post-vaccination side effects (from mild to severe), as well as an action guideline for each symptom. The flow diagram for this fast-track protocol is summarized in
Fig. 1.
Fig. 1
ED fast-track protocol for patients with adverse events after vaccination.
ED = emergency department, PCR = polymerized chain reaction, PVCZ = post-vaccination cohort zone.
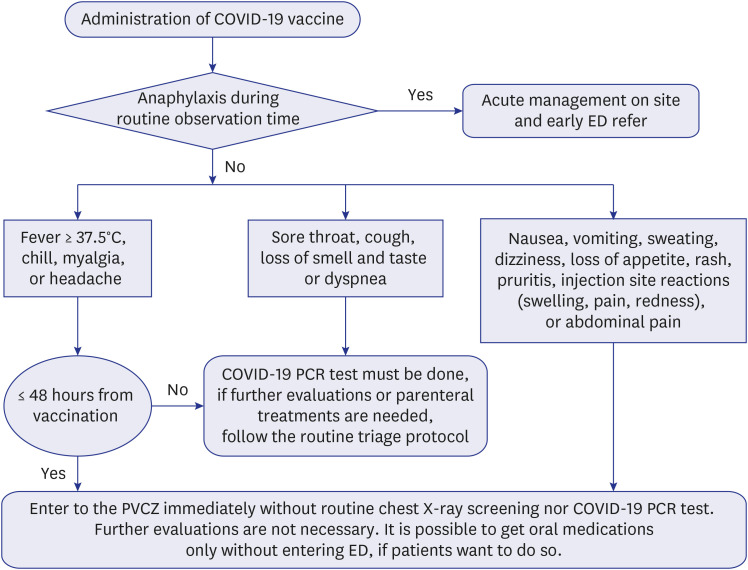

Assuming that most patients in the PVCZ would report similar symptoms and no special tests would be required, two prescription order sets, for symptomatic treatment (in-hospital, and discharge) were created. The in-hospital prescription order set included acetaminophen (AAP), non-steroidal anti-inflammatory drugs (both parenteral and oral form were optional), and a fluid for intravenous hydration. The discharge order set included an oral form of AAP, a muscle relaxant, a pro-kinetic, and an anti-emetic agent. Physicians could freely modify the prescription according to their preferences.
Data collections
We collected data on participants' sex, age, occupation, date of vaccination, and types of vaccine administered. Additionally, we reviewed the electronic medical records of HCWs who visited the ED during the study period and registered their time of visit (arrival and discharge), chief complaints and accompanying symptoms, assigned zone, vital signs, discharge outcomes (home, admission, or death), ED length of stay (LOS), and any oral medication prescribed upon hospital discharge. If patients received diagnostic evaluation or treatment, we collected information on whether any intravenous hydration, or parenteral medication were prescribed, and on whether they had undergone laboratory diagnostic studies, imaging studies, or COVID-19 polymerized chain reaction (PCR) testing. We also reviewed ED visitors' outpatient clinic records (of both scheduled and unscheduled visits) upon discharge. We subdivided HCWs into five groups according to their occupations (physicians, nurses, dentists, pharmacists and other occupations), assuming that there might be a difference in ED visits according to participants' medical knowledge.
Statistical analysis
Categorical variables were compared using Pearson's χ2 test and Fisher's exact test. Participants' baseline characteristics (including sex, age, and occupation) were compared between the group who visited the ED and the group who did not. P values lower than 0.05 were considered statistically significant. The data were analyzed using SPSS v25.0 software (IBM Co., Armonk, NY, USA).
Ethics statements
The Institutional Review Board (IRB) of Ajou University Hospital approved this study and waived the requirement for informed consent (IRB No. AJIR-MED-MDB-21-139).
RESULTS
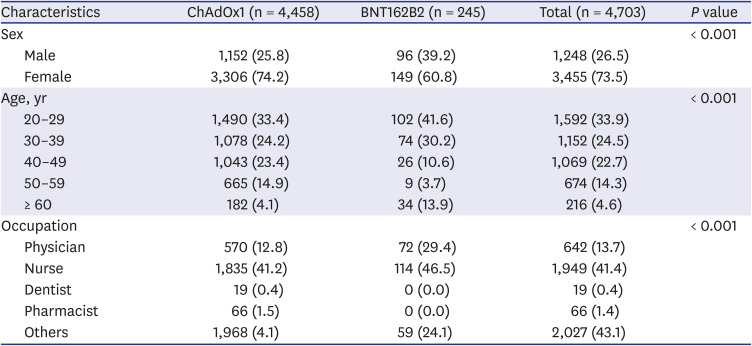
A total of 4,703 in-hospital HCWs received the first dose of either the ChAdOx1 (n = 4,458) vaccine or the BNT162B2 (n = 245) vaccine during the study period. The baseline characteristics of the two vaccinated groups are shown in
Table 1. Overall, 73.5% of HCWs were female and 81.1% were under 50 years of age. Participants between 20 and 29 years old accounted for the largest group, (n = 1,592; 33.9%). Regarding occupations, physicians and nurses comprised 13.7% and 41.4%, respectively. The ‘other occupations’ group accounted for 43.1% of participants; it included nursing assistants, office workers, medical technicians, social workers, and security guards.
Table 1
Baseline characteristics of the two vaccination groups
|
Characteristics |
ChAdOx1 (n = 4,458) |
BNT162B2 (n = 245) |
Total (n = 4,703) |
P value |
|
Sex |
|
|
|
< 0.001 |
|
Male |
1,152 (25.8) |
96 (39.2) |
1,248 (26.5) |
|
Female |
3,306 (74.2) |
149 (60.8) |
3,455 (73.5) |
|
Age, yr |
|
|
|
< 0.001 |
|
20–29 |
1,490 (33.4) |
102 (41.6) |
1,592 (33.9) |
|
30–39 |
1,078 (24.2) |
74 (30.2) |
1,152 (24.5) |
|
40–49 |
1,043 (23.4) |
26 (10.6) |
1,069 (22.7) |
|
50–59 |
665 (14.9) |
9 (3.7) |
674 (14.3) |
|
≥ 60 |
182 (4.1) |
34 (13.9) |
216 (4.6) |
|
Occupation |
|
|
|
< 0.001 |
|
Physician |
570 (12.8) |
72 (29.4) |
642 (13.7) |
|
Nurse |
1,835 (41.2) |
114 (46.5) |
1,949 (41.4) |
|
Dentist |
19 (0.4) |
0 (0.0) |
19 (0.4) |
|
Pharmacist |
66 (1.5) |
0 (0.0) |
66 (1.4) |
|
Others |
1,968 (4.1) |
59 (24.1) |
2,027 (43.1) |

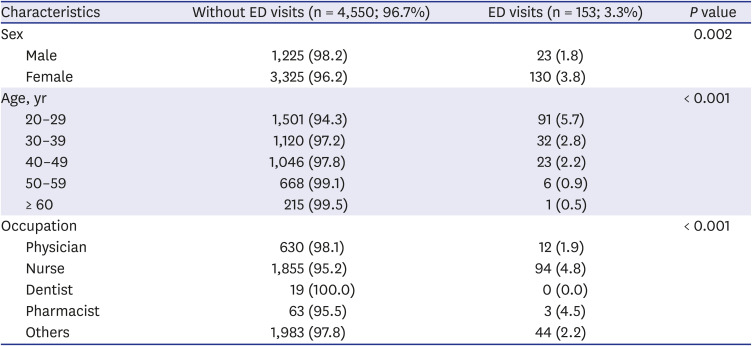
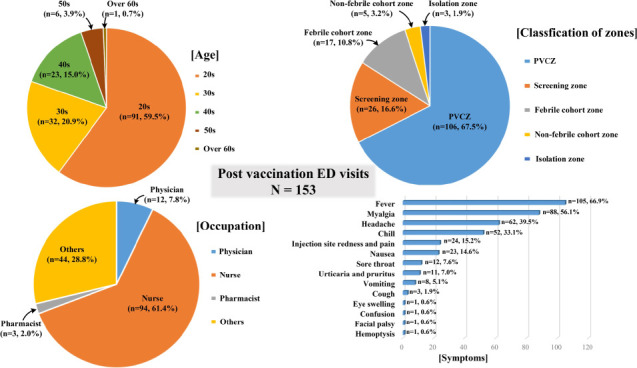
Table 2 shows the baseline characteristics of individuals who visited the ED and of those who did not. A total of 153 patients (3.3%) visited the ED due to adverse events; among them, there were significantly more female patients, compared with male patients (3.8% vs. 1.8%). The age group that visited the ED the most comprised people in their 20s (n = 91; 5.7%); the older the HCWs were, the less they were to visit the ED. Regarding participants’ occupation, 4.8% of nurses, 1.3% of physicians, and 2.2% of individuals from other occupations (non-medical staff), visited the ED.
Table 2
The baseline characteristics of ED visits
|
Characteristics |
Without ED visits (n = 4,550; 96.7%) |
ED visits (n = 153; 3.3%) |
P value |
|
Sex |
|
|
0.002 |
|
Male |
1,225 (98.2) |
23 (1.8) |
|
Female |
3,325 (96.2) |
130 (3.8) |
|
Age, yr |
|
|
< 0.001 |
|
20–29 |
1,501 (94.3) |
91 (5.7) |
|
30–39 |
1,120 (97.2) |
32 (2.8) |
|
40–49 |
1,046 (97.8) |
23 (2.2) |
|
50–59 |
668 (99.1) |
6 (0.9) |
|
≥ 60 |
215 (99.5) |
1 (0.5) |
|
Occupation |
|
|
< 0.001 |
|
Physician |
630 (98.1) |
12 (1.9) |
|
Nurse |
1,855 (95.2) |
94 (4.8) |
|
Dentist |
19 (100.0) |
0 (0.0) |
|
Pharmacist |
63 (95.5) |
3 (4.5) |
|
Others |
1,983 (97.8) |
44 (2.2) |

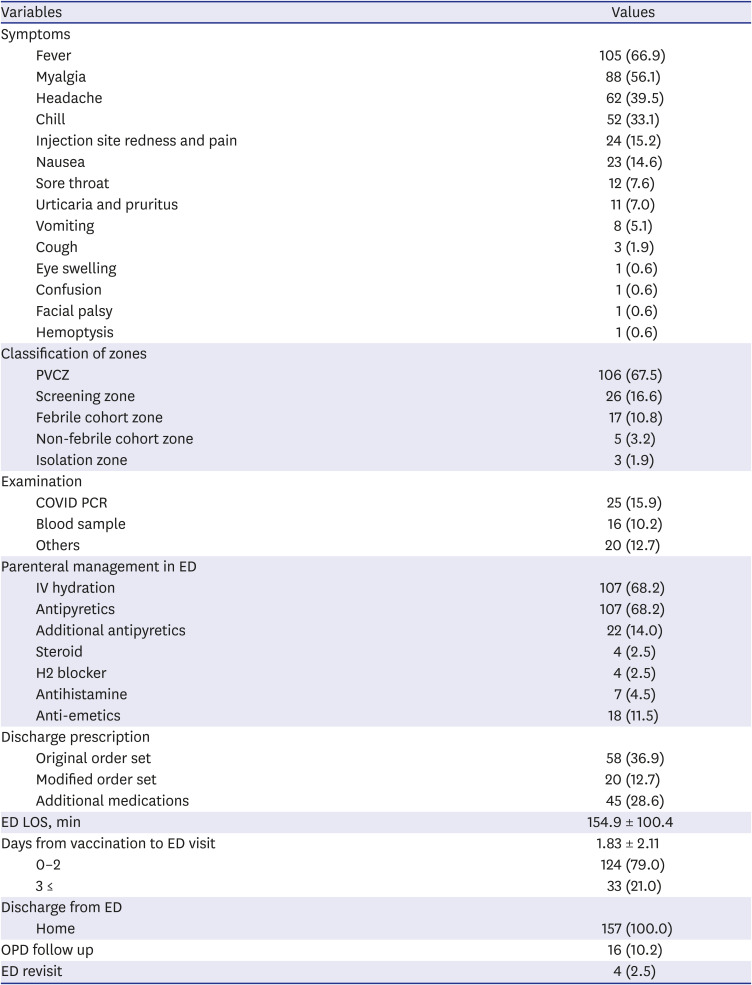
The symptoms most commonly reported by patients who visited the ED post-vaccination were fever (66.9%), myalgia (56.1%), headache (39.5%), chills (33.1%), and redness and pain at the injection site (15.2%), (
Table 3). One patient complained of peripheral type facial palsy, while another reported hemoptysis. There were 11 patients who presented urticaria and pruritus; however, no patient presented anaphylaxis. As a result of zone assignment, 67.5% patients were assigned to the PVCZ, and 26 (16.6%) were discharged from the screening zone before entering the ED. Seventeen cases were assigned to the febrile cohort zone, and while three cases required isolation. Twenty-five cases underwent COVID PCR tests, of which 13 reported experiencing one or more respiratory symptoms, 11 had fever over 48 hours, and one was suspected to have pneumonia due to symptoms of hemoptysis. As parenteral management, intravenous hydration was performed for 68.2% of cases, while antipyretics and anti-emetics were needed for 11.5% of cases. A prescription for a preapproved discharge order set (both original, and modified form), was prescribed for approximately 50% of cases. The mean ED LOS was 154.9 ± 100.4 minutes; the mean LOS for each ED zone is shown in
Supplementary Table 1. Except for the patients who were discharged directly in the screening zone, patients assigned to the PVCZ had lower LOS compared to those in other zones. The mean number of days from vaccination to ED visit were 1.83, whereas 79% of cases arrived visited the ED within two days. No hospitalization was required, however, outpatient follow-up was needed for 16 cases (10.2%) and 4 patients visited the ED at least two times.
Table 3
Common symptoms of ED visitors and their treatment results
|
Variables |
Values |
|
Symptoms |
|
|
Fever |
105 (66.9) |
|
Myalgia |
88 (56.1) |
|
Headache |
62 (39.5) |
|
Chill |
52 (33.1) |
|
Injection site redness and pain |
24 (15.2) |
|
Nausea |
23 (14.6) |
|
Sore throat |
12 (7.6) |
|
Urticaria and pruritus |
11 (7.0) |
|
Vomiting |
8 (5.1) |
|
Cough |
3 (1.9) |
|
Eye swelling |
1 (0.6) |
|
Confusion |
1 (0.6) |
|
Facial palsy |
1 (0.6) |
|
Hemoptysis |
1 (0.6) |
|
Classification of zones |
|
|
PVCZ |
106 (67.5) |
|
Screening zone |
26 (16.6) |
|
Febrile cohort zone |
17 (10.8) |
|
Non-febrile cohort zone |
5 (3.2) |
|
Isolation zone |
3 (1.9) |
|
Examination |
|
|
COVID PCR |
25 (15.9) |
|
Blood sample |
16 (10.2) |
|
Others |
20 (12.7) |
|
Parenteral management in ED |
|
|
IV hydration |
107 (68.2) |
|
Antipyretics |
107 (68.2) |
|
Additional antipyretics |
22 (14.0) |
|
Steroid |
4 (2.5) |
|
H2 blocker |
4 (2.5) |
|
Antihistamine |
7 (4.5) |
|
Anti-emetics |
18 (11.5) |
|
Discharge prescription |
|
|
Original order set |
58 (36.9) |
|
Modified order set |
20 (12.7) |
|
Additional medications |
45 (28.6) |
|
ED LOS, min |
154.9 ± 100.4 |
|
Days from vaccination to ED visit |
1.83 ± 2.11 |
|
0–2 |
124 (79.0) |
|
3 ≤ |
33 (21.0) |
|
Discharge from ED |
|
|
Home |
157 (100.0) |
|
OPD follow up |
16 (10.2) |
|
ED revisit |
4 (2.5) |

DISCUSSION
The Korean government's COVID-19 citizen vaccination program has recently begun, and preferentially targeting individuals ≥ 65 years old, individuals with chronic respiratory disease, and teachers of daycare centers and elementary schools. As the vaccination program is gradually expanding its target population, the concerns and anxiety of the general population regarding the reported adverse events of the vaccines are increasing. Some members of the Korean Society of Emergency Medicine raised concerns that this public anxiety could eventually increase the number of ED visitors and cause difficulties in emergency patient care. However, although many studies of adverse events of vaccinations exist,
46 studies on the post-vaccination utilization of the ED are exceedingly rare. Fertel et al.'s study
8 briefly reported 380 ED visitors (due to reactions to COVID-19 vaccination) over 7 weeks, whereas 70.3% of cases required laboratory work, 61.6% were given intravenous medications, and 17.9% were hospitalized. Jeon et al.
6 studied at a single center in Korea; they adapted a mobile reporting system for adverse events for 1,503 vaccinated HCWs; among them, 994 cases of adverse events were reported, whereas 6 participants visited the ED.
Owing to the limited nationwide supply of the BNT162B2 vaccine (compared to the ChAdOx1), in-hospital HCWs with a higher chance of contact with COVID-19 confirmed patients were given priority for the BNT162B2 vaccine, as it requires a shorter interval between doses and has relatively a higher antibody response rate than the ChAdOx1 vaccine.
910 Only two HCWs (5%) who received the BNT162B2 vaccine visited the ED; they were both nurses in their 30s and visited the ED one day after being vaccinated. One male patient visited the ED due to pain at the injection site; he was prescribed with a discharge order set at the screening zone and stayed for 39 minutes. One female patient visited the ED with symptoms of fever and myalgia and was prescribed a modified discharge order set and parenteral antipyretics; she remained for 30 minutes in the non-febrile zone, as it was forbidden to give parenteral medication in the screening zone.
We categorized the participants' occupations because we assumed that medical staff members would visit the ED less frequently, as they have sufficient medical knowledge to understand adverse vaccination events. However, as shown in
Table 2, medical staff members' visits to the ED were not less frequent, compared with non-medical staff members and the nurse group was the most likely to visit the ED. Approximately 60% of nurses from the BNT162B2 vaccinated group and 80% from the ChAdOx1 vaccinated group were in the age range of 20–39 years (
Supplementary Fig. 1). This 20-39 years of age group is well-reported worldwide as having a higher incidence rate of adverse events due to their stronger immune system, compared with older age groups.
411
We found that 66.9% of ED visitors reported febrile symptoms, and their mean body temperature was 38.2 ± 0.83°C. Febrile symptoms are routinely checked to screen for COVID-19 in most hospitals. Therefore, in the future stages of the vaccination program, which will target larger sectors of the population, many vaccinated people presenting febrile symptoms will visit EDs. Therefore, most EDs' isolation spaces will be saturated. According to Bae et al.,
4 most types of adverse reactions in the ChAdOx1 group tended to decrease in the older age groups. Thus far, vaccination has been focused on older individuals; therefore, it is expected that the prevalence of adverse events will increase when large-scale public vaccination starts. Therefore, it is necessary to prepare a space accordingly.
We preemptively designated and prepared the PVCZ and created the appropriate order sets. The PVCZ was used for 80.9% of patients who entered the ED, except for those discharged directly at the screening zone. Use of the PVCZ was implemented to minimize the burden of patients visiting due to adverse vaccination events on the care provided to all other patients. When patients presented respiratory symptoms or fever that persisted after 48 hours, a COVID-19 PCR test was performed in 15.9% of cases (
Table 3); all tests were negative. Although both of the intra-hospital HCW groups showed various differences in sex, age, and occupation (as shown in
Table 1), we believe that they followed similar infection control protocols throughout the hospital during their working hours and in their daily activities during the COVID-19 pandemic.
It is noteworthy that hospitalization was not required upon discharge from ED. The cases in which outpatient treatment was required included patients experiencing a severe local reaction at the injection site, facial palsy, and visits for urticarial follow-up. A 35-year-old patient reported a peripheral type of facial palsy on the left, with a feeling of swelling in her upper lip within 5 minutes after vaccination. Neurologic examination revealed no other abnormal findings. After consultation with the otolaryngology department, the patient was discharged without medication. At the outpatient visit, one week later, the symptoms showed complete improvement. A 23-year-old woman reporting hemoptysis showed no abnormalities in laboratory tests or in chest computed tomography angiography (including the airway, vessels, mediastinum, and lung parenchyma). A week later, she was scheduled for a follow up at the outpatient clinic, but she did not visit. In both cases, it was difficult to determine an association between symptoms and the administration of the vaccine. In most cases, there were no serious adverse events, and symptomatic treatment was considered the most important in many cases.
This study had a few limitations. First, we did not include data on the overall adverse events of the first dose of the COVID-19 vaccine in-hospital HCWs. Thus, it was difficult to compare how the adverse events of individuals who did not visit the ED differed from those of individuals who did. Second, by analyzing a sample of individuals who only received the first dose of the vaccine, we could not determine clearly how the second dose compared to the first. Third, it was difficult to identify whether the application of the fast-track protocol mitigated overcrowding and treatment delay. Fourth, more than 80% of HCWs were under the age of 50 (
Table 1); this age group generally has a lower chance of having serious underlying medical conditions. Therefore, the utilization trend of the ED from the results of this study might not reflect the real-world circumstances where individuals might have various underlying medical conditions. Finally, due to HCWs controlled epidemiology (as they employ relatively similar self-care measures to mitigate the risk of infection), it was possible to gather them in the PVCZ even if they presented febrile symptoms. However, it might be difficult to ensure that there would be no risk of cross-infection in the PVCZ for the general public.
In conclusion, approximately 3.3% of HCWs who were vaccinated with the first dose of the COVID-19 vaccine visited the ED. We found that the incidence of fever was high, whereas the possibility of serious adverse events was low. As visits to the ED due to adverse events of the COVID-19 vaccine are expected to increase, it is necessary to designate a specific space and treatment protocol for them to minimize the impact on other patients' treatment.







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download